- Enumeration Methods
- Detection Methods
- Demonstration of Capability (DOC)
- Media QC
- Validation/Verification
-

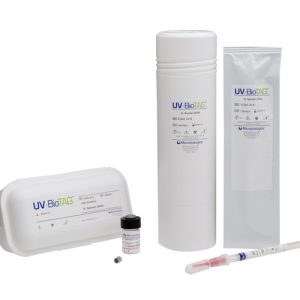 QC microorganisms with Green Fluorescent Protein (GFP) markers It’s not uncommon for food samples to inadvertently become contaminated with control strains used in the laboratory. This leads to false-positive results which can have severe financial and regulatory implications. UV-BioTAG, our line of reference strains containing green fluorescent protein (GFP) markers, make it easy to distinguish standard microorganism strains from laboratory control strains. Optimised for highly visible fluorescence, UV-BioTAG strains provide better stability because the GFP marker is integrated into the chromosome rather than the plasmid. With UV-BioTAG, you can quickly and reliably establish whether a positive test result can be traced back to a control strain cross-contamination issue, or if it is from another source. Applications & Test Methods
QC microorganisms with Green Fluorescent Protein (GFP) markers It’s not uncommon for food samples to inadvertently become contaminated with control strains used in the laboratory. This leads to false-positive results which can have severe financial and regulatory implications. UV-BioTAG, our line of reference strains containing green fluorescent protein (GFP) markers, make it easy to distinguish standard microorganism strains from laboratory control strains. Optimised for highly visible fluorescence, UV-BioTAG strains provide better stability because the GFP marker is integrated into the chromosome rather than the plasmid. With UV-BioTAG, you can quickly and reliably establish whether a positive test result can be traced back to a control strain cross-contamination issue, or if it is from another source. Applications & Test Methods -

 Qualitative control strains for QC testing It doesn’t get much easier than the LYFO-DISK from Microbiologics®. Simply rehydrate LYFO DISK qualitative microorganism pellets with a diluent, such as phosphate buffer or saline, and you’re ready to inoculate your culture media. LYFO DISK, packaged in a glass vial containing 6 pellets of a single strain, offers a ton of flexibility. It's an ideal solution for many quality control applications including presence/absence testing, microbial identification methods, antimicrobial susceptibility testing, media QC, water testing and more! Applications & Test Methods
Qualitative control strains for QC testing It doesn’t get much easier than the LYFO-DISK from Microbiologics®. Simply rehydrate LYFO DISK qualitative microorganism pellets with a diluent, such as phosphate buffer or saline, and you’re ready to inoculate your culture media. LYFO DISK, packaged in a glass vial containing 6 pellets of a single strain, offers a ton of flexibility. It's an ideal solution for many quality control applications including presence/absence testing, microbial identification methods, antimicrobial susceptibility testing, media QC, water testing and more! Applications & Test Methods- Culture Purposes
- Media and reagent QC
- QC of Antimicrobial Susceptibility Test
- QC of microbial identification including biochemical, PCR and rapid molecular methods
- Water tests (e.g. enzyme substrates)
- Disinfectant studies
- Verifications and Validations
- Proficiency tests
- Daily QC
-

 Qualitative control strains as Certified Reference Material for ISO 17025 accredited labs Increasing regulatory oversight in food, pharmaceutical and water industries has prompted many microbiology labs to seek accreditation to ISO 17025 standards. The Lab-Elite CRM, from Microbiologics®, is designed to help those labs meet the requirements of this standard which specify that “reference materials shall, where possible, be traceable to SI units of measurements, or to Certified Reference Materials (section 5.6.3.2)”. Lab-Elite CRM microorganisms are just one passage from the reference culture and are delivered in our signature KWIK-STIK format. As a Certified Reference Material, Lab-Elite comes with a comprehensive Certificate of Analysis detailing the strains identity and characteristics. Applications & Test Methods
Qualitative control strains as Certified Reference Material for ISO 17025 accredited labs Increasing regulatory oversight in food, pharmaceutical and water industries has prompted many microbiology labs to seek accreditation to ISO 17025 standards. The Lab-Elite CRM, from Microbiologics®, is designed to help those labs meet the requirements of this standard which specify that “reference materials shall, where possible, be traceable to SI units of measurements, or to Certified Reference Materials (section 5.6.3.2)”. Lab-Elite CRM microorganisms are just one passage from the reference culture and are delivered in our signature KWIK-STIK format. As a Certified Reference Material, Lab-Elite comes with a comprehensive Certificate of Analysis detailing the strains identity and characteristics. Applications & Test Methods- ISO 17025 laboratories requiring CRM
- Validation/verification of processes and instruments
- Presence/Absence
- Identification methods
-

 Helping microbiologists spread culture since 1986 Introducing KWIK-STICK, from Microbiologics®. Laboratories throughout the world have been praising the KWIK-STIK for decades. That’s because its simple, all-inclusive design makes life easier for lab techs while reducing chances for errors. Each KWIK-STIK contains a qualitative lyophilised microorganism pellet, ampoule of hydrating fluid and inoculating swab. Everything you need to grow reference cultures for QC testing is included in this one handy device. Available in packs of 2 or 6, KWIK-STIKs are universally used for all types of microbiological control testing Applications & Test Methods
Helping microbiologists spread culture since 1986 Introducing KWIK-STICK, from Microbiologics®. Laboratories throughout the world have been praising the KWIK-STIK for decades. That’s because its simple, all-inclusive design makes life easier for lab techs while reducing chances for errors. Each KWIK-STIK contains a qualitative lyophilised microorganism pellet, ampoule of hydrating fluid and inoculating swab. Everything you need to grow reference cultures for QC testing is included in this one handy device. Available in packs of 2 or 6, KWIK-STIKs are universally used for all types of microbiological control testing Applications & Test Methods- Culture Purposes
Daily QC[/fusion_li_item]
- Verification and Validation
- QC of Identification Systems and Assay Kits
- QC of Antimicrobial Susceptibility Test
-

 Quantitative spore-forming QC microorganisms for spoilage detection methods Introducing EZ-SPORE, from Microbiologics®. Spore-forming pathogens can present unique challenges for food laboratories that are conducting tests to detect these contaminants. That’s why food microbiology labs across the globe rely on EZ-SPORE quantitative microorganism preparations for validating their spoilage detection methods or for disinfectant qualification studies. EZ-SPORE preparations provide a concentration of 104 CFU per pellet, making microbiological challenge testing for spore-formers quicker and easier. Applications & Test Methods
Quantitative spore-forming QC microorganisms for spoilage detection methods Introducing EZ-SPORE, from Microbiologics®. Spore-forming pathogens can present unique challenges for food laboratories that are conducting tests to detect these contaminants. That’s why food microbiology labs across the globe rely on EZ-SPORE quantitative microorganism preparations for validating their spoilage detection methods or for disinfectant qualification studies. EZ-SPORE preparations provide a concentration of 104 CFU per pellet, making microbiological challenge testing for spore-formers quicker and easier. Applications & Test Methods- Daily food process controls
- Spoilage detection methods
- Disinfectant Qualification Studies
-

 Certified Reference Material The ultimate in authenticity. Epower CRM quantitative quality control microorganisms, from Microbiologics®, are just one passage from the reference strain. If your lab is ISO 17025 accredited, Epower CRM can help you meet the requirements of Section 5.6.3.2 regarding reference materials. This product offers plenty of flexibility, which is a huge benefit in the lab. Each microorganism pellet has a pre-determined range of CFUs, so you can easily manipulate the product to deliver your desired concentration. You can also create a mixed microorganism population by combining multiple Epower CRM strains. As a Certified Reference Material, Epower CRM comes with a comprehensive Certificate of Analysis detailing the strains identity, characteristics and standard deviation. Applications & Test Methods
Certified Reference Material The ultimate in authenticity. Epower CRM quantitative quality control microorganisms, from Microbiologics®, are just one passage from the reference strain. If your lab is ISO 17025 accredited, Epower CRM can help you meet the requirements of Section 5.6.3.2 regarding reference materials. This product offers plenty of flexibility, which is a huge benefit in the lab. Each microorganism pellet has a pre-determined range of CFUs, so you can easily manipulate the product to deliver your desired concentration. You can also create a mixed microorganism population by combining multiple Epower CRM strains. As a Certified Reference Material, Epower CRM comes with a comprehensive Certificate of Analysis detailing the strains identity, characteristics and standard deviation. Applications & Test Methods- ISO 17025 laboratories requiring CRM
- Detection and enumeration methods
- Verification/Validation
- Bioburden determination
- Minimal lethal concentration
- Disinfectant qualification
- Water tests
- Proficiency tests
- Methods requiring a specific CFU range
- Mixed microorganism population challenge

