-

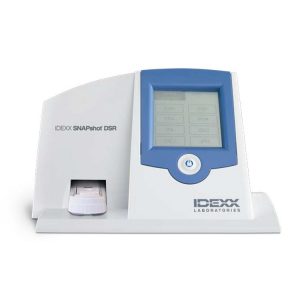 Quickly reads and displays test results Enter data quickly Touch-screen display with numeric data entry, or full alphanumeric data entry with SNAPconnect software or an attached keyboard. Get a clearer picture Upgraded vision system and display make results easy to read. Choose your language Use the SNAPshot DSR display in any of these languages: English, French, German, Spanish, Italian, Portuguese, Chinese, Japanese
Quickly reads and displays test results Enter data quickly Touch-screen display with numeric data entry, or full alphanumeric data entry with SNAPconnect software or an attached keyboard. Get a clearer picture Upgraded vision system and display make results easy to read. Choose your language Use the SNAPshot DSR display in any of these languages: English, French, German, Spanish, Italian, Portuguese, Chinese, Japanese -

 Certified Reference Material The ultimate in authenticity. Epower CRM quantitative quality control microorganisms, from Microbiologics®, are just one passage from the reference strain. If your lab is ISO 17025 accredited, Epower CRM can help you meet the requirements of Section 5.6.3.2 regarding reference materials. This product offers plenty of flexibility, which is a huge benefit in the lab. Each microorganism pellet has a pre-determined range of CFUs, so you can easily manipulate the product to deliver your desired concentration. You can also create a mixed microorganism population by combining multiple Epower CRM strains. As a Certified Reference Material, Epower CRM comes with a comprehensive Certificate of Analysis detailing the strains identity, characteristics and standard deviation. Applications & Test Methods
Certified Reference Material The ultimate in authenticity. Epower CRM quantitative quality control microorganisms, from Microbiologics®, are just one passage from the reference strain. If your lab is ISO 17025 accredited, Epower CRM can help you meet the requirements of Section 5.6.3.2 regarding reference materials. This product offers plenty of flexibility, which is a huge benefit in the lab. Each microorganism pellet has a pre-determined range of CFUs, so you can easily manipulate the product to deliver your desired concentration. You can also create a mixed microorganism population by combining multiple Epower CRM strains. As a Certified Reference Material, Epower CRM comes with a comprehensive Certificate of Analysis detailing the strains identity, characteristics and standard deviation. Applications & Test Methods- ISO 17025 laboratories requiring CRM
- Detection and enumeration methods
- Verification/Validation
- Bioburden determination
- Minimal lethal concentration
- Disinfectant qualification
- Water tests
- Proficiency tests
- Methods requiring a specific CFU range
- Mixed microorganism population challenge
-

 Quantitative spore-forming QC microorganisms for spoilage detection methods Introducing EZ-SPORE, from Microbiologics®. Spore-forming pathogens can present unique challenges for food laboratories that are conducting tests to detect these contaminants. That’s why food microbiology labs across the globe rely on EZ-SPORE quantitative microorganism preparations for validating their spoilage detection methods or for disinfectant qualification studies. EZ-SPORE preparations provide a concentration of 104 CFU per pellet, making microbiological challenge testing for spore-formers quicker and easier. Applications & Test Methods
Quantitative spore-forming QC microorganisms for spoilage detection methods Introducing EZ-SPORE, from Microbiologics®. Spore-forming pathogens can present unique challenges for food laboratories that are conducting tests to detect these contaminants. That’s why food microbiology labs across the globe rely on EZ-SPORE quantitative microorganism preparations for validating their spoilage detection methods or for disinfectant qualification studies. EZ-SPORE preparations provide a concentration of 104 CFU per pellet, making microbiological challenge testing for spore-formers quicker and easier. Applications & Test Methods- Daily food process controls
- Spoilage detection methods
- Disinfectant Qualification Studies
-

 Helping microbiologists spread culture since 1986 Introducing KWIK-STICK, from Microbiologics®. Laboratories throughout the world have been praising the KWIK-STIK for decades. That’s because its simple, all-inclusive design makes life easier for lab techs while reducing chances for errors. Each KWIK-STIK contains a qualitative lyophilised microorganism pellet, ampoule of hydrating fluid and inoculating swab. Everything you need to grow reference cultures for QC testing is included in this one handy device. Available in packs of 2 or 6, KWIK-STIKs are universally used for all types of microbiological control testing Applications & Test Methods
Helping microbiologists spread culture since 1986 Introducing KWIK-STICK, from Microbiologics®. Laboratories throughout the world have been praising the KWIK-STIK for decades. That’s because its simple, all-inclusive design makes life easier for lab techs while reducing chances for errors. Each KWIK-STIK contains a qualitative lyophilised microorganism pellet, ampoule of hydrating fluid and inoculating swab. Everything you need to grow reference cultures for QC testing is included in this one handy device. Available in packs of 2 or 6, KWIK-STIKs are universally used for all types of microbiological control testing Applications & Test Methods- Culture Purposes
Daily QC[/fusion_li_item]
- Verification and Validation
- QC of Identification Systems and Assay Kits
- QC of Antimicrobial Susceptibility Test
-

 Qualitative control strains as Certified Reference Material for ISO 17025 accredited labs Increasing regulatory oversight in food, pharmaceutical and water industries has prompted many microbiology labs to seek accreditation to ISO 17025 standards. The Lab-Elite CRM, from Microbiologics®, is designed to help those labs meet the requirements of this standard which specify that “reference materials shall, where possible, be traceable to SI units of measurements, or to Certified Reference Materials (section 5.6.3.2)”. Lab-Elite CRM microorganisms are just one passage from the reference culture and are delivered in our signature KWIK-STIK format. As a Certified Reference Material, Lab-Elite comes with a comprehensive Certificate of Analysis detailing the strains identity and characteristics. Applications & Test Methods
Qualitative control strains as Certified Reference Material for ISO 17025 accredited labs Increasing regulatory oversight in food, pharmaceutical and water industries has prompted many microbiology labs to seek accreditation to ISO 17025 standards. The Lab-Elite CRM, from Microbiologics®, is designed to help those labs meet the requirements of this standard which specify that “reference materials shall, where possible, be traceable to SI units of measurements, or to Certified Reference Materials (section 5.6.3.2)”. Lab-Elite CRM microorganisms are just one passage from the reference culture and are delivered in our signature KWIK-STIK format. As a Certified Reference Material, Lab-Elite comes with a comprehensive Certificate of Analysis detailing the strains identity and characteristics. Applications & Test Methods- ISO 17025 laboratories requiring CRM
- Validation/verification of processes and instruments
- Presence/Absence
- Identification methods
-

 Qualitative control strains for QC testing It doesn’t get much easier than the LYFO-DISK from Microbiologics®. Simply rehydrate LYFO DISK qualitative microorganism pellets with a diluent, such as phosphate buffer or saline, and you’re ready to inoculate your culture media. LYFO DISK, packaged in a glass vial containing 6 pellets of a single strain, offers a ton of flexibility. It's an ideal solution for many quality control applications including presence/absence testing, microbial identification methods, antimicrobial susceptibility testing, media QC, water testing and more! Applications & Test Methods
Qualitative control strains for QC testing It doesn’t get much easier than the LYFO-DISK from Microbiologics®. Simply rehydrate LYFO DISK qualitative microorganism pellets with a diluent, such as phosphate buffer or saline, and you’re ready to inoculate your culture media. LYFO DISK, packaged in a glass vial containing 6 pellets of a single strain, offers a ton of flexibility. It's an ideal solution for many quality control applications including presence/absence testing, microbial identification methods, antimicrobial susceptibility testing, media QC, water testing and more! Applications & Test Methods- Culture Purposes
- Media and reagent QC
- QC of Antimicrobial Susceptibility Test
- QC of microbial identification including biochemical, PCR and rapid molecular methods
- Water tests (e.g. enzyme substrates)
- Disinfectant studies
- Verifications and Validations
- Proficiency tests
- Daily QC
-

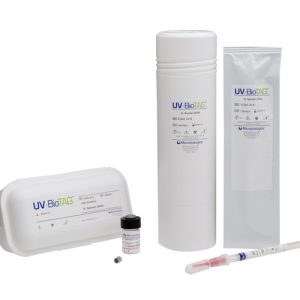 QC microorganisms with Green Fluorescent Protein (GFP) markers It’s not uncommon for food samples to inadvertently become contaminated with control strains used in the laboratory. This leads to false-positive results which can have severe financial and regulatory implications. UV-BioTAG, our line of reference strains containing green fluorescent protein (GFP) markers, make it easy to distinguish standard microorganism strains from laboratory control strains. Optimised for highly visible fluorescence, UV-BioTAG strains provide better stability because the GFP marker is integrated into the chromosome rather than the plasmid. With UV-BioTAG, you can quickly and reliably establish whether a positive test result can be traced back to a control strain cross-contamination issue, or if it is from another source. Applications & Test Methods
QC microorganisms with Green Fluorescent Protein (GFP) markers It’s not uncommon for food samples to inadvertently become contaminated with control strains used in the laboratory. This leads to false-positive results which can have severe financial and regulatory implications. UV-BioTAG, our line of reference strains containing green fluorescent protein (GFP) markers, make it easy to distinguish standard microorganism strains from laboratory control strains. Optimised for highly visible fluorescence, UV-BioTAG strains provide better stability because the GFP marker is integrated into the chromosome rather than the plasmid. With UV-BioTAG, you can quickly and reliably establish whether a positive test result can be traced back to a control strain cross-contamination issue, or if it is from another source. Applications & Test Methods- Enumeration Methods
- Detection Methods
- Demonstration of Capability (DOC)
- Media QC
- Validation/Verification
-

 Solid Phase Cytometry meets Artificial Intelligence TECHNOPATH partners with Microbs for their fully automated solution. To boost productivity in your microbial QC lab, Microbs launches IAN®, an Ultra Rapid Microbiological Method allowing an accurate enumeration of bacteria, yeast and mould within minutes, not days! With IAN®, you can redesign your quality control. No need to wait days to check the microbiological quality of your production. IAN® gives you a quantitative measurement of your contamination in 15 minutes using our innovative direct non-growth detection method.
Solid Phase Cytometry meets Artificial Intelligence TECHNOPATH partners with Microbs for their fully automated solution. To boost productivity in your microbial QC lab, Microbs launches IAN®, an Ultra Rapid Microbiological Method allowing an accurate enumeration of bacteria, yeast and mould within minutes, not days! With IAN®, you can redesign your quality control. No need to wait days to check the microbiological quality of your production. IAN® gives you a quantitative measurement of your contamination in 15 minutes using our innovative direct non-growth detection method.- Mobile Laboratory - Autonomous, its fits where you need it.
- Speed - Time to result: 15 minutes, no enrichment needed.
- Simplicity - No expertise required
- Accuracy - Detection range from 1 to ≈ 5 000 microorganisms/volume analysed
- Liquid sample - Sampling from 100µL to 200mL
- Traceability - Full Data integrity, 21 CFR 11 compliant
-

 Protein Enzyme-Linked Immunosorbent Assay (ELISA) Tests for allergen proteins. The Neogen Allergen Protein ELISA Kits utilise a sandwich ELISA. The target protein present in the sample react with the specific antibodies, which have been adsorbed to the surface of polystyrene microtiter wells. After the removal of unbound proteins by washing, antibodies conjugated with horseradish peroxidase (HRP) are added. This enzyme-labeled antibody forms complexes with the previously bound target protein. Following a second washing step, the enzyme bound to the immunosorbent is detected by the addition of a chromogenic substrate, 3,3’,5,5’-tetramethylbenzidine (TMB). The colour development from this enzymatic reaction varies directly with the concentration of target protein in the sample tested; thus, the absorbance, at 450 nm, is a measure of the concentration of the target protein in the test sample. The quantity of protein in the test sample can be extrapolated from the standard curve, constructed from standards of known concentration, and adjusted to consider the sample dilution.
Protein Enzyme-Linked Immunosorbent Assay (ELISA) Tests for allergen proteins. The Neogen Allergen Protein ELISA Kits utilise a sandwich ELISA. The target protein present in the sample react with the specific antibodies, which have been adsorbed to the surface of polystyrene microtiter wells. After the removal of unbound proteins by washing, antibodies conjugated with horseradish peroxidase (HRP) are added. This enzyme-labeled antibody forms complexes with the previously bound target protein. Following a second washing step, the enzyme bound to the immunosorbent is detected by the addition of a chromogenic substrate, 3,3’,5,5’-tetramethylbenzidine (TMB). The colour development from this enzymatic reaction varies directly with the concentration of target protein in the sample tested; thus, the absorbance, at 450 nm, is a measure of the concentration of the target protein in the test sample. The quantity of protein in the test sample can be extrapolated from the standard curve, constructed from standards of known concentration, and adjusted to consider the sample dilution. -

 Lateral flow devices for specific allergen detection Streamline your process and simplify your allergen testing with 3Neogen Allergen Protein Rapid Tests – qualitative immunochromatographic assays for rapid in-plant monitoring of specific food allergens. Designed for accurate detection of processed and unprocessed allergen proteins, these fast, easy tests can be used for clean-in-place (CIP) final rinse water, environmental swab samples, raw ingredients and finished food products. We’ve taken the hassle out of allergen testing. You see the results.
Lateral flow devices for specific allergen detection Streamline your process and simplify your allergen testing with 3Neogen Allergen Protein Rapid Tests – qualitative immunochromatographic assays for rapid in-plant monitoring of specific food allergens. Designed for accurate detection of processed and unprocessed allergen proteins, these fast, easy tests can be used for clean-in-place (CIP) final rinse water, environmental swab samples, raw ingredients and finished food products. We’ve taken the hassle out of allergen testing. You see the results. -

 Detects as little as 3 µg of protein on surfaces and in hard to reach areas CleanTrace™ ALLTEC60 Surface Protein Swabs are a fast and easy way to visually check whether cleaning has been carried out to a satisfactory standard by means of protein detection. By comparing the colour of the swab and solution against the label, the user can easily interpret the results for the presence of allergens. Clean-Trace® ALTEC60 Surface Protein (Allergen) Test Swab is validated for a range of allergenic proteins, including egg, milk, gluten, soy, peanut, almond and buckwheat. Refrigeration is not required -swabs can be stored at room temperature (25°C or 77°F). This easy-to-use device requires minimal training. The results are semi-quantitative with four possible color interpretations. The faster the test turns purple and the stronger the color change the higher the level of contamination on a surface. Quick detection and confirmation allows immediate corrective action to take place. A visual reading of a colour change indicates the level of cleanliness by detecting as little as 3µg of allergen on surfaces and in solution. No instrumentation is required to interpret the sample results. This product is easily integrated into company HACCP programs to monitor for cleaning effectiveness. Its ease of use, reliable detection and fast, visible results can help in teaching staff the importance of cleaning.
Detects as little as 3 µg of protein on surfaces and in hard to reach areas CleanTrace™ ALLTEC60 Surface Protein Swabs are a fast and easy way to visually check whether cleaning has been carried out to a satisfactory standard by means of protein detection. By comparing the colour of the swab and solution against the label, the user can easily interpret the results for the presence of allergens. Clean-Trace® ALTEC60 Surface Protein (Allergen) Test Swab is validated for a range of allergenic proteins, including egg, milk, gluten, soy, peanut, almond and buckwheat. Refrigeration is not required -swabs can be stored at room temperature (25°C or 77°F). This easy-to-use device requires minimal training. The results are semi-quantitative with four possible color interpretations. The faster the test turns purple and the stronger the color change the higher the level of contamination on a surface. Quick detection and confirmation allows immediate corrective action to take place. A visual reading of a colour change indicates the level of cleanliness by detecting as little as 3µg of allergen on surfaces and in solution. No instrumentation is required to interpret the sample results. This product is easily integrated into company HACCP programs to monitor for cleaning effectiveness. Its ease of use, reliable detection and fast, visible results can help in teaching staff the importance of cleaning. -

 Ensure pipettes are performing properly and operator skills are standardized Manage your pipette calibration program and assess operator competency. Used in combination with the Artel PCS, the PCS Software ensures your pipettes and their operators are working together to generate accurate and reproducible results. The PCS Software provides scheduling for pipette calibrations and interim performance verifications; complete documentation and pipette inventory management, even for pipettes calibrated outside of PCS Software – and it’s ideal for standardising pipetting technique and assessing operator competency.
Ensure pipettes are performing properly and operator skills are standardized Manage your pipette calibration program and assess operator competency. Used in combination with the Artel PCS, the PCS Software ensures your pipettes and their operators are working together to generate accurate and reproducible results. The PCS Software provides scheduling for pipette calibrations and interim performance verifications; complete documentation and pipette inventory management, even for pipettes calibrated outside of PCS Software – and it’s ideal for standardising pipetting technique and assessing operator competency.

