- Antimicrobial Effectiveness Testing - USP <51>
- Aseptic Processing Environment - USP <1116>
- Disinfectant Qualification - USP <116>
- Growth Promotion Testing - USP <61>, <62>, <71>
- Suitability Testing - USP <51>, <61>, <62>, <71>
- Validation of Neutralisation Methods - USP <1227>
- Water for Pharmaceutical Purposes - USP <1231>
-

 Microbial Controls made from your Environmental Isolates Tracking and trending environmental microbial isolates is a growing concern for pharmaceutical, nutraceutical, medical device, personal care product and food manufacturing laboratories. Microbiologics offers a comprehensive program for environmental isolate management. Our team of experts will identify, characterise, preserve and manufacture your strain into a test-ready control format for ongoing QC. Partner with TECHNOPATH and Microbiologics to reduce cost, minimize risk and increase confidence in your environmental monitoring program. Applications & Test Methods
Microbial Controls made from your Environmental Isolates Tracking and trending environmental microbial isolates is a growing concern for pharmaceutical, nutraceutical, medical device, personal care product and food manufacturing laboratories. Microbiologics offers a comprehensive program for environmental isolate management. Our team of experts will identify, characterise, preserve and manufacture your strain into a test-ready control format for ongoing QC. Partner with TECHNOPATH and Microbiologics to reduce cost, minimize risk and increase confidence in your environmental monitoring program. Applications & Test Methods -

 Two passage reference cultures in our signature KWIK-STIK format Introducing KWIK-STICK Plus, from Microbiologics®. Some laboratory applications require strains that are a fewer passages from the original reference culture. For those special instances, we offer KWIK-STIK Plus, which combines microorganisms that are just two passages from their reference strains, with our signature KWIK-STIK format. Each device features a qualitative lyophilised microorganism pellet, ampoule of hydrating fluid and inoculating swab; so everything you need for growing control cultures is included in one handy device. Applications & Test Methods
Two passage reference cultures in our signature KWIK-STIK format Introducing KWIK-STICK Plus, from Microbiologics®. Some laboratory applications require strains that are a fewer passages from the original reference culture. For those special instances, we offer KWIK-STIK Plus, which combines microorganisms that are just two passages from their reference strains, with our signature KWIK-STIK format. Each device features a qualitative lyophilised microorganism pellet, ampoule of hydrating fluid and inoculating swab; so everything you need for growing control cultures is included in one handy device. Applications & Test Methods- Antibiotic assays
- Instrument validations
- Validation of neutralization methods
- Water tests
- Disinfectant qualification
-

 Ready-to-Use Microorganisms for Antimicrobial Effectiveness and Preservative Efficacy Challenge Testing Microbiologists are no strangers to the fact that safety and effectiveness are crucial elements of developing pharmaceutical and personal care products. The EZ-PEC™ quantitative microorganisms, from Microbiologics®, help laboratories conduct Antimicrobial Effectiveness and Preservative Efficacy testing with convenience and confidence. Following a few simple steps, EZ-PEC™ delivers a final concentration of 1.0 x 105 to 1.0 x 106 CFU per ml of the product being tested. It’s a simple solution that can save you time and resources while helping you meet Pharmacopeia and other standards and guidelines. Applications & Test Methods
Ready-to-Use Microorganisms for Antimicrobial Effectiveness and Preservative Efficacy Challenge Testing Microbiologists are no strangers to the fact that safety and effectiveness are crucial elements of developing pharmaceutical and personal care products. The EZ-PEC™ quantitative microorganisms, from Microbiologics®, help laboratories conduct Antimicrobial Effectiveness and Preservative Efficacy testing with convenience and confidence. Following a few simple steps, EZ-PEC™ delivers a final concentration of 1.0 x 105 to 1.0 x 106 CFU per ml of the product being tested. It’s a simple solution that can save you time and resources while helping you meet Pharmacopeia and other standards and guidelines. Applications & Test Methods- Antimicrobial Effectiveness Testing
- Preservative Efficacy Testing
- Applications requiring a high CFU concentration
-

 Ready-to-Use Quantitative QC Microorganisms for Growth Promotion Testing Introducing EZ-CFU One Step, from Microbiologics®. This product is designed specifically for Growth Promotion Testing, Suitability of Tests for Specified Microorganisms, Suitability of Counting Methods and more. These ready-to-use quantitative microorganisms are just 3 passages from the reference culture and deliver 10-100 CFU per inoculum, as required by the Pharmacopeias, with minimal prep time. Applications & Test Methods
Ready-to-Use Quantitative QC Microorganisms for Growth Promotion Testing Introducing EZ-CFU One Step, from Microbiologics®. This product is designed specifically for Growth Promotion Testing, Suitability of Tests for Specified Microorganisms, Suitability of Counting Methods and more. These ready-to-use quantitative microorganisms are just 3 passages from the reference culture and deliver 10-100 CFU per inoculum, as required by the Pharmacopeias, with minimal prep time. Applications & Test Methods- Growth Promotion Testing
- Media Challenge Testing
- Suitability of Counting Methods
- Suitability of Sterility Tests
- Suitability of Tests for Specified Microorganisms
- Microbial Limits Testing
- Microbial Enumeration Testing
- Validation of Neutralisation Methods
- Methods requiring a low CFU concentration
-

 Economical Quantitative QC Microorganisms for Growth Promotion Testing EZ-CFU, from Microbiologics®, ready-to-use reference cultures for Growth Promotion Testing. Delivering 10-100 CFU per 0.1 ml inoculum, following a simple 1:10 dilution step, each microorganism suspension provides up to 90 tests. These handy kits allow for fast, reliable Growth Promotion Testing. Plus, EZ-CFU microorganisms are just three passages from the reference strains. All in all, EZ-CFU makes meeting Pharmacopeia standards for Growth Promotion Testing easier than ever. Applications & Test Methods
Economical Quantitative QC Microorganisms for Growth Promotion Testing EZ-CFU, from Microbiologics®, ready-to-use reference cultures for Growth Promotion Testing. Delivering 10-100 CFU per 0.1 ml inoculum, following a simple 1:10 dilution step, each microorganism suspension provides up to 90 tests. These handy kits allow for fast, reliable Growth Promotion Testing. Plus, EZ-CFU microorganisms are just three passages from the reference strains. All in all, EZ-CFU makes meeting Pharmacopeia standards for Growth Promotion Testing easier than ever. Applications & Test Methods- Growth Promotion Testing
- Media Challenge Testing
- Suitability of Counting Methods
- Suitability of Sterility Tests
- Suitability of Tests for Specified Microorganisms
- Microbial Limits Testing
- Microbial Enumeration Testing
- Validation of Neutralisation Methods
- Methods requiring a low CFU concentration
-

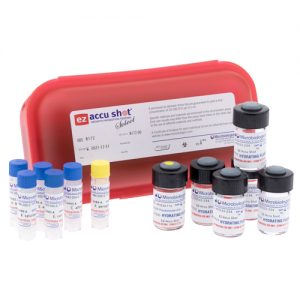 Five compendial GPT strains, plus E. coli – all in one convenient kit EZ-Accu Shot Select, from Microbiologics®, contains the 5 compendial organisms for Growth Promotion Testing, plus E. coli for a total of 6 strains in one convenient kit. Perfect for qualifying EZ-Accu Shot for use in your laboratory, the EZ-Accu Shot Select kit contains one instant dissolve pellet of each of the 6 strains and 6 vials of hydration fluid. Each pellet delivers 10-100 CFU per inoculum with no serial dilutions and provided 8 hours of stability after rehydration for added flexibility to perform tests. EZ-Accu Shot Select strains are just 3 passages from the reference culture to meet Pharmacopeia standards. Strains Include:
Five compendial GPT strains, plus E. coli – all in one convenient kit EZ-Accu Shot Select, from Microbiologics®, contains the 5 compendial organisms for Growth Promotion Testing, plus E. coli for a total of 6 strains in one convenient kit. Perfect for qualifying EZ-Accu Shot for use in your laboratory, the EZ-Accu Shot Select kit contains one instant dissolve pellet of each of the 6 strains and 6 vials of hydration fluid. Each pellet delivers 10-100 CFU per inoculum with no serial dilutions and provided 8 hours of stability after rehydration for added flexibility to perform tests. EZ-Accu Shot Select strains are just 3 passages from the reference culture to meet Pharmacopeia standards. Strains Include:- Staphylococcus aureus subsp. aureus derived from ATCC® 6538™*
- Pseudomonas aeruginosa derived from ATCC® 9027™*
- Bacillus subtilis subsp. spizizenii derived from ATCC® 6633™*
- Candida albicans derived from ATCC® 10231™*
- Aspergillus brasiliensis derived from ATCC® 16404™*
- Escherichia coli derived from ATCC® 8739™*
-

 Growth Promotion Testing EZ-Accu Shot, from Microbiologics®, provides a fast, convenient and reliable solution for Growth Promotion Testing, Media Challenge Testing and more. Each instant-dissolve microorganism pellet is designed to deliver 10-100 CFU per inoculum and provides 8 hours of stability after rehydration, so you can meet Pharmacopeia guidelines with ease. Applications & Test Methods
Growth Promotion Testing EZ-Accu Shot, from Microbiologics®, provides a fast, convenient and reliable solution for Growth Promotion Testing, Media Challenge Testing and more. Each instant-dissolve microorganism pellet is designed to deliver 10-100 CFU per inoculum and provides 8 hours of stability after rehydration, so you can meet Pharmacopeia guidelines with ease. Applications & Test Methods- Growth Promotion Testing
- Media Challenge Testing
- Suitability of Counting Methods
- Suitability of Sterility Tests
- Suitability of Tests for Specified Microorganisms
- Microbial Limits Testing
- Microbial Enumeration Testing
- Validation of Neutralization Methods
- Methods requiring a low CFU concentration

