- Non-infectious and replication deficient, enables safe handling in contrast to viral samples
- Fully-extractable with a real viral protein coat; superior to “naked” transcribed RNA
- Long shelf life: 2-year stability at 2 - 8°C
-

 On-going Performance Monitoring for Routine Patient Testing AccuPlex Monkeypox Positive Reference Material Kit is a full-process quality solution designed to challenge the molecular test procedure from extraction to detection, ensuring clinical laboratories can have confidence in their assay results. The product contains positive materials directed against targets for Monkeypox assays.
On-going Performance Monitoring for Routine Patient Testing AccuPlex Monkeypox Positive Reference Material Kit is a full-process quality solution designed to challenge the molecular test procedure from extraction to detection, ensuring clinical laboratories can have confidence in their assay results. The product contains positive materials directed against targets for Monkeypox assays. -

 SeraCare’s SARS-CoV-2 Quality Solution AccuPlex™ SARS-CoV-2 Reference Material, from SeraCare, is designed to measure day-to-day performance of the assay, providing both a positive and a negative reference solution. The product is available in versions that offer different SARS-CoV-2 sequence coverage: Version 1 covers the CDC and WHO consensus sequences, Version 2 has been enhanced to also include the S gene region, and Version 3 is expanded to include the full SARS‐CoV‐2 viral genome (Table 1). All products also include negative reference materials targeting the human RNase P gene. AccuPlex™ SARS-CoV-2 Verification Panel, coupled with the AccuPlex™ SARS-CoV-2 Reference Material, is a complete Quality Solution. Both products are available in three versions with different SARS-CoV-2 sequence coverage: Version 1 covers the CDC and WHO consensus sequences, Version 2 has been enhanced to also include the S gene region, and Version 3 is expanded to include the full SARS-CoV-2 viral genome (Table 1). All products also include negative reference materials targeting the human RNase P gene.
SeraCare’s SARS-CoV-2 Quality Solution AccuPlex™ SARS-CoV-2 Reference Material, from SeraCare, is designed to measure day-to-day performance of the assay, providing both a positive and a negative reference solution. The product is available in versions that offer different SARS-CoV-2 sequence coverage: Version 1 covers the CDC and WHO consensus sequences, Version 2 has been enhanced to also include the S gene region, and Version 3 is expanded to include the full SARS‐CoV‐2 viral genome (Table 1). All products also include negative reference materials targeting the human RNase P gene. AccuPlex™ SARS-CoV-2 Verification Panel, coupled with the AccuPlex™ SARS-CoV-2 Reference Material, is a complete Quality Solution. Both products are available in three versions with different SARS-CoV-2 sequence coverage: Version 1 covers the CDC and WHO consensus sequences, Version 2 has been enhanced to also include the S gene region, and Version 3 is expanded to include the full SARS-CoV-2 viral genome (Table 1). All products also include negative reference materials targeting the human RNase P gene.- Non-infectious and replication deficient, enables safe handling of positive material
- Fully-extractable with a real viral protein coat; serves as a full-process reference material
- Compatible with assays targeting CDC and WHO consensus sequences
- Includes negative reference material for targeting sequences for the human RNAse P gene
- 2 year stability at 2 - 8°C
- Customisable to sequences of interest to meet unique assay design requirements
-

 Clinical Diagnostic Quality Solutions for SARS-CoV-2 Variant Analysis AccuPlex SARS-CoV-2 Variant Panel 1 contains the full SARS-CoV-2 genomic RNA with a focus on S-gene mutations (Table 1) in three prominent variants of concern (VOC): UK variant B.1.1.7, South Africa variant B.1.351, and Brazil variant P.1. The kit has one vial for each variant as well as a fourth wild type (reference sequence NC_045512) control vial. An RNase P negative control vial is also included.
Clinical Diagnostic Quality Solutions for SARS-CoV-2 Variant Analysis AccuPlex SARS-CoV-2 Variant Panel 1 contains the full SARS-CoV-2 genomic RNA with a focus on S-gene mutations (Table 1) in three prominent variants of concern (VOC): UK variant B.1.1.7, South Africa variant B.1.351, and Brazil variant P.1. The kit has one vial for each variant as well as a fourth wild type (reference sequence NC_045512) control vial. An RNase P negative control vial is also included.- Performance confirmation of existing SARS-CoV-2 molecular assays with different variants
- Reference materials for SARS-CoV-2 variant genotyping assays
- Reference materials for SARS-CoV-2 NGS assays
- Non-infectious and replication deficient, enables safe handling of positive material
- Fully extractable with a real viral protein coat; superior to “naked” transcribed RNA
- 2 year stability at 2 - 8°C
-

 Full Genome Coverage now available AccuPlex™ SARS-CoV-2 Verification Panel, from SeraCare, is optimised for assay verification at installation by documenting test performance along the assay’s range enabling laboratories to establish lower limits of detection, perform assay comparisons, and evaluate staff proficiency. The product contains positive materials including the full SARS-CoV-2 viral genome, and negative materials targeting the human RNase P gene. AccuPlex™ SARS-CoV-2 Verification Panel, coupled with the AccuPlex™ SARS-CoV-2 Reference Material, is a complete Quality Solution designed to challenge the entire molecular test procedure ensuring clinical laboratories can have confidence in their SARS-CoV-2 assay results.
Full Genome Coverage now available AccuPlex™ SARS-CoV-2 Verification Panel, from SeraCare, is optimised for assay verification at installation by documenting test performance along the assay’s range enabling laboratories to establish lower limits of detection, perform assay comparisons, and evaluate staff proficiency. The product contains positive materials including the full SARS-CoV-2 viral genome, and negative materials targeting the human RNase P gene. AccuPlex™ SARS-CoV-2 Verification Panel, coupled with the AccuPlex™ SARS-CoV-2 Reference Material, is a complete Quality Solution designed to challenge the entire molecular test procedure ensuring clinical laboratories can have confidence in their SARS-CoV-2 assay results.- Non-infectious and replication deficient, enables safe handling of positive material
- Fully-extractable with a real viral protein coat; serves as a full-process reference material
- Compatible with assays targeting CDC and WHO consensus sequences
- Includes negative reference material for targeting sequences for the human RNAse P gene
- 2 year stability at 2 - 8°C
- Customisable to sequences of interest to meet unique assay design requirements
-

 Measure day-to-day performance AccuPlex™ SARS-CoV-2, Flu A/B and RSV Reference Material Kit, from SeraCare, is designed to measure day-to-day performance of the assay, providing both a positive and a negative reference solution. Designed for use with multiplexed molecular assays that can detect SARS-CoV-2, influenza A/B and respiratory syncytial virus (RSV). AccuPlex™ SARS-CoV-2, Flu A/B and RSV Reference Material Kit, coupled with the AccuPlex™ SARS-CoV-2, Flu A/B and RSV Verification Panel, is a complete Quality Solution designed to challenge the entire molecular test procedure ensuring clinical laboratories can have confidence in their multiplex assay results.
Measure day-to-day performance AccuPlex™ SARS-CoV-2, Flu A/B and RSV Reference Material Kit, from SeraCare, is designed to measure day-to-day performance of the assay, providing both a positive and a negative reference solution. Designed for use with multiplexed molecular assays that can detect SARS-CoV-2, influenza A/B and respiratory syncytial virus (RSV). AccuPlex™ SARS-CoV-2, Flu A/B and RSV Reference Material Kit, coupled with the AccuPlex™ SARS-CoV-2, Flu A/B and RSV Verification Panel, is a complete Quality Solution designed to challenge the entire molecular test procedure ensuring clinical laboratories can have confidence in their multiplex assay results.- Non-infectious and replication deficient, enables safe handling of positive material
- Fully-extractable with a real viral protein coat; serves as a full-process reference material
- 2 year stability at 2 - 8°C
- Optimised for assay verification and day-to-day performance monitoring
-

 Have confidence in your multiplex assay results for SARS-CoV-2 & Flu A/B AccuPlex™ SARS-CoV-2, Flu A/B and RSV Verification Panel, from SeraCare, is optimised for assay verification at installation by documenting test performance along the assay’s range, enabling laboratories to establish lower limits of detection, perform assay comparisons, and evaluate staff proficiency. Designed for use with multiplexed molecular assays that can detect SARS-CoV-2, influenza A/B and respiratory syncytial virus (RSV). AccuPlex™ SARS-CoV-2, Flu A/B and RSV Verification Panel, coupled with the AccuPlex™ SARS-CoV-2, Flu A/B and RSV Reference Material Kit, is a complete Quality Solution designed to challenge the entire molecular test procedure ensuring clinical laboratories can have confidence in their multiplex assay results.
Have confidence in your multiplex assay results for SARS-CoV-2 & Flu A/B AccuPlex™ SARS-CoV-2, Flu A/B and RSV Verification Panel, from SeraCare, is optimised for assay verification at installation by documenting test performance along the assay’s range, enabling laboratories to establish lower limits of detection, perform assay comparisons, and evaluate staff proficiency. Designed for use with multiplexed molecular assays that can detect SARS-CoV-2, influenza A/B and respiratory syncytial virus (RSV). AccuPlex™ SARS-CoV-2, Flu A/B and RSV Verification Panel, coupled with the AccuPlex™ SARS-CoV-2, Flu A/B and RSV Reference Material Kit, is a complete Quality Solution designed to challenge the entire molecular test procedure ensuring clinical laboratories can have confidence in their multiplex assay results.- Non-infectious and replication deficient, enables safe handling of positive material
- Fully-extractable with a real viral protein coat; serves as a full-process reference material
- 2 year stability at 2 - 8°C
- Optimised for assay verification and day-to-day performance monitoring
-

 Ensure antibody testing accuracy and performance To support the many SARS-CoV-2 serology assay formats available, the kit is currently offered in two configurations, one designed for IgG-specific assays and the other for total antibody tests. Series 1000 is released using the Abbott ARCHITECT SARS-CoV-2 IgG assay, and Series 2000 is released using the Roche cobas® Elecsys® Anti-SARS-CoV-2 assay. Reference materials are specifically formulated to exhibit low positive reactivity in true patient-like matrices, to test assay performance near critical signal cutoff boundaries. AccuSet™ SARS-CoV-2 Performance Panel is intended to provide an out-of-the-box solution to evaluate SARS-CoV-2 antibody detection assays with highly characterised human specimens whether generating validation data for a regulatory submission or performing assay verification in a clinical laboratory setting.
Ensure antibody testing accuracy and performance To support the many SARS-CoV-2 serology assay formats available, the kit is currently offered in two configurations, one designed for IgG-specific assays and the other for total antibody tests. Series 1000 is released using the Abbott ARCHITECT SARS-CoV-2 IgG assay, and Series 2000 is released using the Roche cobas® Elecsys® Anti-SARS-CoV-2 assay. Reference materials are specifically formulated to exhibit low positive reactivity in true patient-like matrices, to test assay performance near critical signal cutoff boundaries. AccuSet™ SARS-CoV-2 Performance Panel is intended to provide an out-of-the-box solution to evaluate SARS-CoV-2 antibody detection assays with highly characterised human specimens whether generating validation data for a regulatory submission or performing assay verification in a clinical laboratory setting. -

 Ensure Strep A Laboratory Testing Assay Performance ACCURUN® reference materials are intended to estimate laboratory testing precision and can be used to detect errors in laboratory testing procedures. ACCURUN Swab Strep A Reference Material is formulated for use with molecular test methods used to detect Streptococcus pyogenes (Strep A). They are designed as whole organism reference materials that can be used to assess accuracy and performance of the full testing process – extraction, amplification, and detection – of molecular assays for Strep A. For Research Use Only. Not for use in diagnostic procedures.
Ensure Strep A Laboratory Testing Assay Performance ACCURUN® reference materials are intended to estimate laboratory testing precision and can be used to detect errors in laboratory testing procedures. ACCURUN Swab Strep A Reference Material is formulated for use with molecular test methods used to detect Streptococcus pyogenes (Strep A). They are designed as whole organism reference materials that can be used to assess accuracy and performance of the full testing process – extraction, amplification, and detection – of molecular assays for Strep A. For Research Use Only. Not for use in diagnostic procedures. -

 Whole-cell or whole-organism external controls Empower your results and challenge your methods with SeraCare’s ACCURUN® molecular controls and reference materials. ACCURUN molecular controls and reference materials are whole-cell or whole-organism external controls that help you monitor all aspects of your molecular testing methods and provide additional confidence in your laboratory test results. A well-designed QC program can help you avoid costly false-negative or false-positive results. ACCURUN molecular controls effectively detect low-positives closer to assay-specific cutoffs, enabling better detection of assay variability. Powerful ACCURUN controls and reference materials help to ensure complete control over assay performance monitoring. Monitors the Entire Testing Process. In order to execute the highest level of quality control, QC methods should be able to monitor the entire testing process, not just a portion. ACCURUN molecular controls are whole-cell or whole-organism controls that can appropriately challenge your assay from extraction through detection.
Whole-cell or whole-organism external controls Empower your results and challenge your methods with SeraCare’s ACCURUN® molecular controls and reference materials. ACCURUN molecular controls and reference materials are whole-cell or whole-organism external controls that help you monitor all aspects of your molecular testing methods and provide additional confidence in your laboratory test results. A well-designed QC program can help you avoid costly false-negative or false-positive results. ACCURUN molecular controls effectively detect low-positives closer to assay-specific cutoffs, enabling better detection of assay variability. Powerful ACCURUN controls and reference materials help to ensure complete control over assay performance monitoring. Monitors the Entire Testing Process. In order to execute the highest level of quality control, QC methods should be able to monitor the entire testing process, not just a portion. ACCURUN molecular controls are whole-cell or whole-organism controls that can appropriately challenge your assay from extraction through detection. -

 Empower your results and challenge your methods with SeraCare’s ACCURUN® serology controls and reference materials. SeraCare's ACCURUN controls and reference materials are designed to be weakly reactive to help monitor your serology assays and provide additional confidence in your laboratory test results. Monitoring your assay performance can help you avoid costly repeats and, more importantly, avoid false-negative and false-positive results. With ACCURUN controls, you can troubleshoot your test methods and isolate system errors in your laboratory. Powerful ACCURUN controls and reference materials help to ensure complete control over assay performance monitoring. Challenges Your Assay More Effectively A low-positive control challenges your assay more than a medium-to-high control since it is closer to the assay cut-off; therefore, any variability in the assay will be easier to detect. Assay monitoring with ACCURUN single and multi-analyte controls improves confidence in your serology test results.
Empower your results and challenge your methods with SeraCare’s ACCURUN® serology controls and reference materials. SeraCare's ACCURUN controls and reference materials are designed to be weakly reactive to help monitor your serology assays and provide additional confidence in your laboratory test results. Monitoring your assay performance can help you avoid costly repeats and, more importantly, avoid false-negative and false-positive results. With ACCURUN controls, you can troubleshoot your test methods and isolate system errors in your laboratory. Powerful ACCURUN controls and reference materials help to ensure complete control over assay performance monitoring. Challenges Your Assay More Effectively A low-positive control challenges your assay more than a medium-to-high control since it is closer to the assay cut-off; therefore, any variability in the assay will be easier to detect. Assay monitoring with ACCURUN single and multi-analyte controls improves confidence in your serology test results. -

 Available for use with serological and molecular assays, SeraCare’s portfolio of AccuSet performance panels contains highly characterized, raw, undiluted plasma specimens collected from unique individuals positive for your analytes of interest. Each panel contains a comprehensive comparative data sheet with test results from a wide variety of leading commercially available assays and platforms. AccuSet performance panels can be used to evaluate assay specificity, sensitivity, repeatability, and reproducibility to assist you in validating new test methods and equipment, run head-to-head assay comparisons, demonstrate lab proficiency, and train laboratory personnel.
Available for use with serological and molecular assays, SeraCare’s portfolio of AccuSet performance panels contains highly characterized, raw, undiluted plasma specimens collected from unique individuals positive for your analytes of interest. Each panel contains a comprehensive comparative data sheet with test results from a wide variety of leading commercially available assays and platforms. AccuSet performance panels can be used to evaluate assay specificity, sensitivity, repeatability, and reproducibility to assist you in validating new test methods and equipment, run head-to-head assay comparisons, demonstrate lab proficiency, and train laboratory personnel. -

 Ensure antibody testing accuracy and performance AccuSet™ SARS-CoV-2 Performance Panel from SeraCare - This eleven-member validation panel consists of undiluted, naturally occurring human-plasma members. Ten panel members represent collections from multiple individuals positive for antibodies to SARS-CoV-2; a single negative member is also included. Test results from commercially available SARS-CoV-2 antibody assays are included for comparative analysis. AccuSet™ SARS-CoV-2 Performance Panel is intended to provide an out-of-the-box solution to evaluate SARS-CoV-2 antibody detection assays with highly characterized human specimens whether generating validation data for a regulatory submission or performing assay verification in a clinical laboratory setting. ACCURUN® Anti-SARS-CoV-2 Reference Material Kit is designed to support assay installation and monitoring of day-to-day assay performance, providing a complete quality solution for SARS-CoV-2 antibody testing.
Ensure antibody testing accuracy and performance AccuSet™ SARS-CoV-2 Performance Panel from SeraCare - This eleven-member validation panel consists of undiluted, naturally occurring human-plasma members. Ten panel members represent collections from multiple individuals positive for antibodies to SARS-CoV-2; a single negative member is also included. Test results from commercially available SARS-CoV-2 antibody assays are included for comparative analysis. AccuSet™ SARS-CoV-2 Performance Panel is intended to provide an out-of-the-box solution to evaluate SARS-CoV-2 antibody detection assays with highly characterized human specimens whether generating validation data for a regulatory submission or performing assay verification in a clinical laboratory setting. ACCURUN® Anti-SARS-CoV-2 Reference Material Kit is designed to support assay installation and monitoring of day-to-day assay performance, providing a complete quality solution for SARS-CoV-2 antibody testing. -
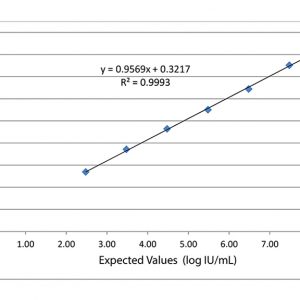
 Validate and Monitor your assays AccuSpan linearity panels from SeraCare are designed to span the dynamic range of quantitative infectious disease assays and evaluate instrumentation analytical sensitivity. Linearity panels effectively challenge assay performance at defined intervals to ensure consistency throughout the entire reportable range. In addition to linearity studies, these panels are useful in validation procedures for new assay implementation, operator training, and troubleshooting signs of assay deterioration.
Validate and Monitor your assays AccuSpan linearity panels from SeraCare are designed to span the dynamic range of quantitative infectious disease assays and evaluate instrumentation analytical sensitivity. Linearity panels effectively challenge assay performance at defined intervals to ensure consistency throughout the entire reportable range. In addition to linearity studies, these panels are useful in validation procedures for new assay implementation, operator training, and troubleshooting signs of assay deterioration. -

 Ensure reagents are operating effectively from lot-to-lot AccuTrak qualification panels from SeraCare are designed as a cost-effective solution to deliver the consistent results you need to gain confidence in your assay’s performance and ensure reagents are operating effectively from lot-to-lot. SeraCare's AccuTrak qualification panels are utilised by clinical laboratories worldwide to help strengthen quality control protocols and procedures for infectious disease diagnostic assays. With products for HIV, hepatitis, CMV, syphilis, HPV, and HTLV, SeraCare offers a comprehensive portfolio to help effectively monitor assay performance. Reliable, Consistent, Cost-Effective Solutions for Your Assay QC Program
Ensure reagents are operating effectively from lot-to-lot AccuTrak qualification panels from SeraCare are designed as a cost-effective solution to deliver the consistent results you need to gain confidence in your assay’s performance and ensure reagents are operating effectively from lot-to-lot. SeraCare's AccuTrak qualification panels are utilised by clinical laboratories worldwide to help strengthen quality control protocols and procedures for infectious disease diagnostic assays. With products for HIV, hepatitis, CMV, syphilis, HPV, and HTLV, SeraCare offers a comprehensive portfolio to help effectively monitor assay performance. Reliable, Consistent, Cost-Effective Solutions for Your Assay QC Program -

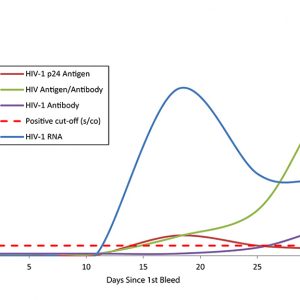 Assess your assay development milestones When your assay development requires natural patient specimens that represent the body’s true response to an infection, you can depend on AccuVert seroconversion panels as a gold standard with which to assess your assay development milestones. The SeraCare seroconversion panels are developed using raw, undiluted plasma collected from a single individual during the development of an infection and subsequent immunological response. Spanning an array of infectious diseases from HIV to hepatitis and syphilis, SeraCare's panels provide you with a diverse selection of products with high-quality datasets to help evaluate your assay. A Rich History in Seroconversion Panels SeraCare have been a trusted provider of seroconversion panels for over 30 years, IVD diagnostic manufacturers worldwide have used our panels in the development and validation of their assays for decades. As shown in the World Health Organisation HIV test evaluation kit reports, SeraCare HIV seroconversion panels have been used for comparative studies for HIV test kit evaluations, and are also frequently referenced in package inserts of leading IVD infectious disease platforms.
Assess your assay development milestones When your assay development requires natural patient specimens that represent the body’s true response to an infection, you can depend on AccuVert seroconversion panels as a gold standard with which to assess your assay development milestones. The SeraCare seroconversion panels are developed using raw, undiluted plasma collected from a single individual during the development of an infection and subsequent immunological response. Spanning an array of infectious diseases from HIV to hepatitis and syphilis, SeraCare's panels provide you with a diverse selection of products with high-quality datasets to help evaluate your assay. A Rich History in Seroconversion Panels SeraCare have been a trusted provider of seroconversion panels for over 30 years, IVD diagnostic manufacturers worldwide have used our panels in the development and validation of their assays for decades. As shown in the World Health Organisation HIV test evaluation kit reports, SeraCare HIV seroconversion panels have been used for comparative studies for HIV test kit evaluations, and are also frequently referenced in package inserts of leading IVD infectious disease platforms. -

 Accelerate assay optimisation, simplify regulatory compliance, and ensure quality. Understand and manage the performance of your automated liquid handlers, multichannel pipettes, labware, operators, and more. Compatible with virtually all automated liquid handling systems and multichannel handheld pipettes, the Artel MVS sits on a mobile workstation for portable rapid calibration, verifications and optimisation of dispensed volumes with high precision and accuracy. The MVS’s unique, dual-dye photometric measurements are robust against environmental influences and traceable to SI units to enable comparison across operators, protocols, equipment, and locations. Using the Calibrator Plate, Verification Plates, and an Artel-certified Plate Reader, the MVS supports an unbroken chain of traceability to national and international standards.
Accelerate assay optimisation, simplify regulatory compliance, and ensure quality. Understand and manage the performance of your automated liquid handlers, multichannel pipettes, labware, operators, and more. Compatible with virtually all automated liquid handling systems and multichannel handheld pipettes, the Artel MVS sits on a mobile workstation for portable rapid calibration, verifications and optimisation of dispensed volumes with high precision and accuracy. The MVS’s unique, dual-dye photometric measurements are robust against environmental influences and traceable to SI units to enable comparison across operators, protocols, equipment, and locations. Using the Calibrator Plate, Verification Plates, and an Artel-certified Plate Reader, the MVS supports an unbroken chain of traceability to national and international standards. -

 Ensure data integrity, reliable test results, and regulatory compliance. Easily calibrate pipettes, perform interim verifications, standardise operator skills, manage pipette inventory, and more with the Artel PCS® Pipette Calibration System. Fast, accurate, and precise, the PCS is a portable and easy-to-use volume verification system that simplifies single-channel pipette calibration, interim volume verification, and pipette user/operator training and competency assessment. Paired with the included PCS Software, the PCS helps you stay on top of your pipette inventory with calibration and interim verification scheduling, email notifications, pipette and pipette operator status, and comprehensive, auditable documentation. Easily meet both external regulatory requirements and internal quality standards to ensure data quality. The power of the PCS stems from the ratiometric photometry technology and standardised dye solutions that are used to measure dispensed volumes. The system is robust against differences in environmental conditions and calibration results are traceable to NIST standards, enabling straightforward comparison of pipettes, operators, methods, and locations.
Ensure data integrity, reliable test results, and regulatory compliance. Easily calibrate pipettes, perform interim verifications, standardise operator skills, manage pipette inventory, and more with the Artel PCS® Pipette Calibration System. Fast, accurate, and precise, the PCS is a portable and easy-to-use volume verification system that simplifies single-channel pipette calibration, interim volume verification, and pipette user/operator training and competency assessment. Paired with the included PCS Software, the PCS helps you stay on top of your pipette inventory with calibration and interim verification scheduling, email notifications, pipette and pipette operator status, and comprehensive, auditable documentation. Easily meet both external regulatory requirements and internal quality standards to ensure data quality. The power of the PCS stems from the ratiometric photometry technology and standardised dye solutions that are used to measure dispensed volumes. The system is robust against differences in environmental conditions and calibration results are traceable to NIST standards, enabling straightforward comparison of pipettes, operators, methods, and locations. -

 Internal Quality Control Software from Technopath Manufacturing Ltd. IAMQC Daily is a comprehensive Internal Quality Control software that applies Westgard and/or any user-defined QC rules to individual QC results. The software automatically builds interactive Levey-Jennings charts and tables and provides summary and detailed customised reports to the end user. IAMQC Daily integrates with Microsoft Excel to produce customised electronic reports. The system also allows the import of pre-defined templates, resulting in instant system setups. Users can create audit trails, action logs and summary reports at the click of a button. IAMQC Daily comprises a centralised program that facilitates the analysis of multiple QC materials, across numerous departments in a laboratory setting. The system can also be integrated with IAMQC Peer to satisfy both Internal and External QC requirements. IAMQC Daily offers a centralised review of all QC data from all laboratories/instruments. Central administrator access facilitates managers to review QC performance at multiple facilities – no need to visit each laboratory site. Closer monitoring of QC from remote locations without additional costs provides a flexible option for managing the inter and intra-lab performance. The software works on an ‘open’ platform that allows the end user to add all types of control material from a range of laboratories. Both IAMQC Daily and Expert can run using an internet OR intranet connection. Each PC license allows the user to manage an unlimited number of departments, control materials (not limited to Technopath materials) and instruments. An unlimited number of user logins can be added to the system at any stage. An administrator module can also manage user logins, customising the functionality that is available to each user. All data can also be filtered using the same logic to apply a user-friendly atmosphere and save time scrolling through data.
Internal Quality Control Software from Technopath Manufacturing Ltd. IAMQC Daily is a comprehensive Internal Quality Control software that applies Westgard and/or any user-defined QC rules to individual QC results. The software automatically builds interactive Levey-Jennings charts and tables and provides summary and detailed customised reports to the end user. IAMQC Daily integrates with Microsoft Excel to produce customised electronic reports. The system also allows the import of pre-defined templates, resulting in instant system setups. Users can create audit trails, action logs and summary reports at the click of a button. IAMQC Daily comprises a centralised program that facilitates the analysis of multiple QC materials, across numerous departments in a laboratory setting. The system can also be integrated with IAMQC Peer to satisfy both Internal and External QC requirements. IAMQC Daily offers a centralised review of all QC data from all laboratories/instruments. Central administrator access facilitates managers to review QC performance at multiple facilities – no need to visit each laboratory site. Closer monitoring of QC from remote locations without additional costs provides a flexible option for managing the inter and intra-lab performance. The software works on an ‘open’ platform that allows the end user to add all types of control material from a range of laboratories. Both IAMQC Daily and Expert can run using an internet OR intranet connection. Each PC license allows the user to manage an unlimited number of departments, control materials (not limited to Technopath materials) and instruments. An unlimited number of user logins can be added to the system at any stage. An administrator module can also manage user logins, customising the functionality that is available to each user. All data can also be filtered using the same logic to apply a user-friendly atmosphere and save time scrolling through data. -

 Select QC Rules and make meaningful QC decisions IAMQC Expert, from Technopath Manufacturing Ltd, is an interactive system that helps front line laboratory staff select QC rules, reduce unnecessary repeats and make meaningful QC decisions. IAMQC Expert allows the end-user to monitor method performance relative to clinical requirements and focus on the tests that require their attention. Times and technologies are changing rapidly. Instruments and methodologies are more accurate, precise and stable than they were a decade ago. Most laboratories have adopted these new technical advances, but few have modified their QC processes to match. Many laboratories are still using a 1-2s rule as recommended by Levey and Jennings in 1951. Technopath Manufacturing Ltd have designed a QC system that will alert users to significant changes and not generate QC flags when the system is operating safely within acceptable limits. The system compares method performance to defined quality requirements (rather than to last month’s data) and recommends QC strategies that will warn users when QC data points exceed acceptable performance - with a minimal number of false flags. In the design of our QC system we “balance” the quality control system to meet the changing performance and stability of the analytical system.
Select QC Rules and make meaningful QC decisions IAMQC Expert, from Technopath Manufacturing Ltd, is an interactive system that helps front line laboratory staff select QC rules, reduce unnecessary repeats and make meaningful QC decisions. IAMQC Expert allows the end-user to monitor method performance relative to clinical requirements and focus on the tests that require their attention. Times and technologies are changing rapidly. Instruments and methodologies are more accurate, precise and stable than they were a decade ago. Most laboratories have adopted these new technical advances, but few have modified their QC processes to match. Many laboratories are still using a 1-2s rule as recommended by Levey and Jennings in 1951. Technopath Manufacturing Ltd have designed a QC system that will alert users to significant changes and not generate QC flags when the system is operating safely within acceptable limits. The system compares method performance to defined quality requirements (rather than to last month’s data) and recommends QC strategies that will warn users when QC data points exceed acceptable performance - with a minimal number of false flags. In the design of our QC system we “balance” the quality control system to meet the changing performance and stability of the analytical system. -

 Innovative, real-time, Peer Comparison Software from Technopath Manufacturing Ltd. The web based system facilitates laboratories testing the same lot number of control material to access valuable information from their colleagues through peer comparison. The reports that are generated in IAMQC Peer compare the accuracy and precision of analytical processes between laboratories and peer groups. This information can be extremely valuable, indicating the user’s performance relative to their peer group and also providing powerful troubleshooting tools when attempting to resolve potential problems. To participate in IAMQC Peer, each individual laboratory submits their individual results or summary statistics (mean, standard deviation, and number of data points) to the central database maintained by Technopath Manufacturing Ltd. Laboratories data may be submitted manually on-line or, alternatively, captured by one of our many interfacing options. The information provided by IAMQC Peer can be used on a monthly basis to evaluate how well lab’s methods are operating, relative to the overall peer group. Users can also look at this peer data in real-time, interactive, tables online, when they are investigating a potential problem with accuracy or precision for an individual method.
Innovative, real-time, Peer Comparison Software from Technopath Manufacturing Ltd. The web based system facilitates laboratories testing the same lot number of control material to access valuable information from their colleagues through peer comparison. The reports that are generated in IAMQC Peer compare the accuracy and precision of analytical processes between laboratories and peer groups. This information can be extremely valuable, indicating the user’s performance relative to their peer group and also providing powerful troubleshooting tools when attempting to resolve potential problems. To participate in IAMQC Peer, each individual laboratory submits their individual results or summary statistics (mean, standard deviation, and number of data points) to the central database maintained by Technopath Manufacturing Ltd. Laboratories data may be submitted manually on-line or, alternatively, captured by one of our many interfacing options. The information provided by IAMQC Peer can be used on a monthly basis to evaluate how well lab’s methods are operating, relative to the overall peer group. Users can also look at this peer data in real-time, interactive, tables online, when they are investigating a potential problem with accuracy or precision for an individual method. -

 Positive multi-marker QC Multichem Infectious Disease (ID) Quality Controls (QC) samples, have been specifically optimised and validated to match assays commonly used for infectious diseases testing. Multichem ID-B is an unassayed control, optimised for Abbott and Diasorin instruments. Scroll down to view the product benefits and analyte list. The Multichem ID Multi-marker range of products is easily identifiable by their colour coding (in this case B for blue). Multichem ID-B consolidates six analytes (see analyte list below). This unassayed product is optimised for specific platforms, including; Abbott Architect, Abbott Alinity i, Abbott Alinity s, DiaSorin Liaison, DiaSorin Liaison XL MUREX.
Positive multi-marker QC Multichem Infectious Disease (ID) Quality Controls (QC) samples, have been specifically optimised and validated to match assays commonly used for infectious diseases testing. Multichem ID-B is an unassayed control, optimised for Abbott and Diasorin instruments. Scroll down to view the product benefits and analyte list. The Multichem ID Multi-marker range of products is easily identifiable by their colour coding (in this case B for blue). Multichem ID-B consolidates six analytes (see analyte list below). This unassayed product is optimised for specific platforms, including; Abbott Architect, Abbott Alinity i, Abbott Alinity s, DiaSorin Liaison, DiaSorin Liaison XL MUREX. -

 Positive quality control for antibodies to SARS-CoV-2 (including IgG) Multichem ID-COVID19 G is an unasssayed third-party quality control for Antibodies to SARS-CoV-2 (including IgG). This Quality Control is available with access to the Technopath Clinical Diagnostics inter-laboratory Peer program, IAMQC®. The Multichem ID-COVID19 G (unassayed) is a positive control for antibodies to the SARS-CoV-2 virus (including IgG). The kit is delivered in a choice of two configurations: 4x2mL tubes with an integrated instrument barcode and secondly 4 x 4mL vials. The product is suitable for use on all major platforms, including amongst others, Abbott, Roche and Siemens. All Multichem ID-COVID19 quality controls are supported by the inter-laboratory Peer program - IAMQC®.
Positive quality control for antibodies to SARS-CoV-2 (including IgG) Multichem ID-COVID19 G is an unasssayed third-party quality control for Antibodies to SARS-CoV-2 (including IgG). This Quality Control is available with access to the Technopath Clinical Diagnostics inter-laboratory Peer program, IAMQC®. The Multichem ID-COVID19 G (unassayed) is a positive control for antibodies to the SARS-CoV-2 virus (including IgG). The kit is delivered in a choice of two configurations: 4x2mL tubes with an integrated instrument barcode and secondly 4 x 4mL vials. The product is suitable for use on all major platforms, including amongst others, Abbott, Roche and Siemens. All Multichem ID-COVID19 quality controls are supported by the inter-laboratory Peer program - IAMQC®. -

 Negative quality control for antibodies to SARS-CoV-2 (including IgG) Multichem ID-COVID19Neg is an unassayed negative third-party quality control for antibodies to SARS-CoV-2 (including IgG). This Quality Control is available with access to the Technopath Clinical Diagnostics inter-laboratory Peer program, IAMQC®. The Multichem ID-COVID19Neg (unassayed) is an unassayed negative third-party quality control for antibodies to SARS-CoV-2 (including IgG). The kit is delivered in a choice of two configurations: 4x2mL tubes with an integrated instrument barcode and secondly 4 x 4mL vials. The product is suitable for use on all major platforms, including amongst others, Abbott, Roche and Siemens. All Multichem ID-COVID19 quality controls are supported by the inter-laboratory Peer program - IAMQC®.
Negative quality control for antibodies to SARS-CoV-2 (including IgG) Multichem ID-COVID19Neg is an unassayed negative third-party quality control for antibodies to SARS-CoV-2 (including IgG). This Quality Control is available with access to the Technopath Clinical Diagnostics inter-laboratory Peer program, IAMQC®. The Multichem ID-COVID19Neg (unassayed) is an unassayed negative third-party quality control for antibodies to SARS-CoV-2 (including IgG). The kit is delivered in a choice of two configurations: 4x2mL tubes with an integrated instrument barcode and secondly 4 x 4mL vials. The product is suitable for use on all major platforms, including amongst others, Abbott, Roche and Siemens. All Multichem ID-COVID19 quality controls are supported by the inter-laboratory Peer program - IAMQC®. -

 Negative multi-marker QC Multichem Infectious Disease (ID) Quality Controls (QC) samples, have been specifically optimised and validated to match assays commonly used for infectious diseases testing. Multichem ID-SeroNeg provides a negative control for the analytes listed below. Scroll down to view the product benefits and analyte list. Multichem ID SeroNeg provides a third party, unassayed, negative control for commonly tested assays for anti-HIV, anti-HCV, anti HTLV, anti-HBc, anti-Treponema, HBsAg and HIVp24
Negative multi-marker QC Multichem Infectious Disease (ID) Quality Controls (QC) samples, have been specifically optimised and validated to match assays commonly used for infectious diseases testing. Multichem ID-SeroNeg provides a negative control for the analytes listed below. Scroll down to view the product benefits and analyte list. Multichem ID SeroNeg provides a third party, unassayed, negative control for commonly tested assays for anti-HIV, anti-HCV, anti HTLV, anti-HBc, anti-Treponema, HBsAg and HIVp24 -
 Multichem A1c Control, from Technopath Manufacturing Ltd, is intended for use as a third party, bi- level, liquid stable quality control material to monitor the precision of laboratory testing procedures for Haemoglobin A1c determination Assays. The intended patient population are those with diabetes. The haemoglobin A1c test, also called HbA1c, glycated haemoglobin test, or glycol-haemoglobin, is an important blood test that shows how well a patient’s diabetes is being controlled. Multichem A1c Control is designed as a bi-level, liquid stable frozen product. The product will typically be run after reagent calibration and at a frequency dictated by laboratory QC procedures and reagent / instrument manufacturer instructions. The product should be treated the same as patient specimens and run in accordance with the instructions accompanying the instrument, kit or reagent being used. The control is to be stored at -20°C to –80°C and thawed as per IFU prior to use. The product is to be stored at 2 to 8°C between use.
Multichem A1c Control, from Technopath Manufacturing Ltd, is intended for use as a third party, bi- level, liquid stable quality control material to monitor the precision of laboratory testing procedures for Haemoglobin A1c determination Assays. The intended patient population are those with diabetes. The haemoglobin A1c test, also called HbA1c, glycated haemoglobin test, or glycol-haemoglobin, is an important blood test that shows how well a patient’s diabetes is being controlled. Multichem A1c Control is designed as a bi-level, liquid stable frozen product. The product will typically be run after reagent calibration and at a frequency dictated by laboratory QC procedures and reagent / instrument manufacturer instructions. The product should be treated the same as patient specimens and run in accordance with the instructions accompanying the instrument, kit or reagent being used. The control is to be stored at -20°C to –80°C and thawed as per IFU prior to use. The product is to be stored at 2 to 8°C between use. -
 Multichem AE (Ammonia Ethanol) Control, from Technopath Manufacturing Ltd, is intended for use as a third party liquid stable quality control material to monitor the precision of laboratory testing procedures for Ammonia and Ethanol Assays. Multichem AE Control is designed as a liquid stable frozen product. The product will typically be run after reagent calibration and at a frequency dictated by laboratory QC procedures and reagent / instrument manufacturer instructions. The product should be treated the same as patient specimens and run in accordance with the instructions accompanying the instrument, kit or reagent being used. The control is to be stored at -20°C to –80°C and thawed as per IFU prior to use. The product is to be stored at 2 to 8°C between use.
Multichem AE (Ammonia Ethanol) Control, from Technopath Manufacturing Ltd, is intended for use as a third party liquid stable quality control material to monitor the precision of laboratory testing procedures for Ammonia and Ethanol Assays. Multichem AE Control is designed as a liquid stable frozen product. The product will typically be run after reagent calibration and at a frequency dictated by laboratory QC procedures and reagent / instrument manufacturer instructions. The product should be treated the same as patient specimens and run in accordance with the instructions accompanying the instrument, kit or reagent being used. The control is to be stored at -20°C to –80°C and thawed as per IFU prior to use. The product is to be stored at 2 to 8°C between use. -

 Multichem AMH Control, from Technopath Manufacturing Ltd, is intended for use as a third party, tri-level, liquid stable quality control material to monitor the precision of laboratory testing procedures for Anti Mullerian Hormone Assays. The product will typically be run to provide a minimum of 2 levels of control to monitor AMH assay performance within the analytical ranges. Multichem AMH Control will be run after reagent calibration and at a frequency dictated by laboratory QC procedures and reagent / instrument manufacturer instructions. The product should be treated the same as patient specimens and run in accordance with the instructions accompanying the instrument, kit or reagent being used. The Control is to be stored at -20°C to –80°C and thawed as per IFU prior to use. The product is to be stored at 2 to 8°C between use.
Multichem AMH Control, from Technopath Manufacturing Ltd, is intended for use as a third party, tri-level, liquid stable quality control material to monitor the precision of laboratory testing procedures for Anti Mullerian Hormone Assays. The product will typically be run to provide a minimum of 2 levels of control to monitor AMH assay performance within the analytical ranges. Multichem AMH Control will be run after reagent calibration and at a frequency dictated by laboratory QC procedures and reagent / instrument manufacturer instructions. The product should be treated the same as patient specimens and run in accordance with the instructions accompanying the instrument, kit or reagent being used. The Control is to be stored at -20°C to –80°C and thawed as per IFU prior to use. The product is to be stored at 2 to 8°C between use. -

 Third party, bi-level, liquid stable, multi-analyte quality control material to monitor the precision of laboratory testing procedures for Cerebral Spinal Fluid Assays. Multichem CSF, from Technopath Manufacturing Ltd, contains 4 analytes including CSF glucose, protein, IgG and lactate. Multichem CSF Controls are designed as bi-level, liquid stable frozen products. These products will typically be run after reagent calibration and at a frequency dictated by laboratory QC procedures and reagent / instrument manufacturer instructions. The products should be treated the same as patient specimens and run in accordance with the instructions accompanying the instrument, kit or reagent being used. The controls are should be stored at -20°C to -80°C and thawed as per IFU prior to use. The products should be stored at 2 to 8°C between use.
Third party, bi-level, liquid stable, multi-analyte quality control material to monitor the precision of laboratory testing procedures for Cerebral Spinal Fluid Assays. Multichem CSF, from Technopath Manufacturing Ltd, contains 4 analytes including CSF glucose, protein, IgG and lactate. Multichem CSF Controls are designed as bi-level, liquid stable frozen products. These products will typically be run after reagent calibration and at a frequency dictated by laboratory QC procedures and reagent / instrument manufacturer instructions. The products should be treated the same as patient specimens and run in accordance with the instructions accompanying the instrument, kit or reagent being used. The controls are should be stored at -20°C to -80°C and thawed as per IFU prior to use. The products should be stored at 2 to 8°C between use. -

 Multichem D-Dimer Control, from Technopath Manufacturing Ltd, is intended for use as a third party, bi-level, liquid stable quality control material to monitor the precision of laboratory testing procedures for D-Dimer Assays. Multichem D-Dimer Control is intended for use as a third party, bi-level, liquid stable quality control material to monitor the precision of laboratory testing procedures for D-Dimer Assays. Multichem D-Dimer Control is designed as a bi-level, liquid stable frozen product. The product will typically be run to provide a minimum of two levels of control to monitor D-Dimer assay performance within the analytical ranges. Multichem D-Dimer Control will be run after reagent calibration and at a frequency dictated by laboratory QC procedures and reagent / instrument manufacturer instructions. The product should be treated the same as patient specimens and run in accordance with the instructions accompanying the instrument, kit or reagent being used. The Control is to be stored at -20°C to -80°C and thawed as per IFU prior to use. The product is to be stored at 2 to 8°C between use.
Multichem D-Dimer Control, from Technopath Manufacturing Ltd, is intended for use as a third party, bi-level, liquid stable quality control material to monitor the precision of laboratory testing procedures for D-Dimer Assays. Multichem D-Dimer Control is intended for use as a third party, bi-level, liquid stable quality control material to monitor the precision of laboratory testing procedures for D-Dimer Assays. Multichem D-Dimer Control is designed as a bi-level, liquid stable frozen product. The product will typically be run to provide a minimum of two levels of control to monitor D-Dimer assay performance within the analytical ranges. Multichem D-Dimer Control will be run after reagent calibration and at a frequency dictated by laboratory QC procedures and reagent / instrument manufacturer instructions. The product should be treated the same as patient specimens and run in accordance with the instructions accompanying the instrument, kit or reagent being used. The Control is to be stored at -20°C to -80°C and thawed as per IFU prior to use. The product is to be stored at 2 to 8°C between use. -
 Multichem hsTn Control, from Technopath Manufacturing Ltd, is intended for use as a third party, single level, liquid stable quality control material to monitor the precision of laboratory testing procedures for high sensitive Troponin Assays. The intended patient population is Cardiac patients, where a requirement has been identified for testing conditions associated with elevated levels of Troponin I and Troponin T. Multichem hsTn Control is designed as a single level, liquid stable frozen product. The product will typically be run as a low level/high sensitive control, in conjunction with one other level of the Multichem IA / Multichem IA Plus series of serum controls for the same analytes, after reagent calibration and at a frequency dictated by laboratory QC procedures and reagent / instrument manufacturer instructions. This product design is based on the existing Multichem IA Plus L1 control, but incorporating the required features of a single low level Troponin Control, to supplement the IA Plus Control to monitor the precision of Troponin assay methods, at the low end of the linear dynamic range. The product should be treated the same as patient specimens and run in accordance with the instructions accompanying the instrument, kit or reagent being used. The control is to be stored at -20°C to –80°C and thawed as per IFU prior to use. The product is to be stored at 2 to 8°C between use.
Multichem hsTn Control, from Technopath Manufacturing Ltd, is intended for use as a third party, single level, liquid stable quality control material to monitor the precision of laboratory testing procedures for high sensitive Troponin Assays. The intended patient population is Cardiac patients, where a requirement has been identified for testing conditions associated with elevated levels of Troponin I and Troponin T. Multichem hsTn Control is designed as a single level, liquid stable frozen product. The product will typically be run as a low level/high sensitive control, in conjunction with one other level of the Multichem IA / Multichem IA Plus series of serum controls for the same analytes, after reagent calibration and at a frequency dictated by laboratory QC procedures and reagent / instrument manufacturer instructions. This product design is based on the existing Multichem IA Plus L1 control, but incorporating the required features of a single low level Troponin Control, to supplement the IA Plus Control to monitor the precision of Troponin assay methods, at the low end of the linear dynamic range. The product should be treated the same as patient specimens and run in accordance with the instructions accompanying the instrument, kit or reagent being used. The control is to be stored at -20°C to –80°C and thawed as per IFU prior to use. The product is to be stored at 2 to 8°C between use. -

 Multichem IA contains 83 analytes including fertility and thyroid hormones, steroid hormones, cardiac markers, anaemia markers, therapeutic drugs, adrenal markers and bone metabolism markers. Multichem IA Control, from Technopath Manufacturing Ltd, is intended for use as a third party, multi-constituent quality control material to monitor the precision of laboratory testing procedures for Immunoassay Assays. Please note: the main difference between Multichem IA Plus and Multichem IA product is the addition of three tumor markers to Multichem IA Plus; CA 125, CA 15-3 and CA 19-9. Multichem IA is designed as a tri-level, liquid stable frozen product. This product will typically be run after reagent calibration and at a frequency dictated by laboratory QC procedures and reagent / instrument manufacturer instructions. The product should be treated the same as patient specimens and run in accordance with the instructions accompanying the instrument, kit or reagent being used. The control should be stored at -20°C to –80°C and thawed as per IFU prior to use. The product should be stored at 2 to 8°C between use.
Multichem IA contains 83 analytes including fertility and thyroid hormones, steroid hormones, cardiac markers, anaemia markers, therapeutic drugs, adrenal markers and bone metabolism markers. Multichem IA Control, from Technopath Manufacturing Ltd, is intended for use as a third party, multi-constituent quality control material to monitor the precision of laboratory testing procedures for Immunoassay Assays. Please note: the main difference between Multichem IA Plus and Multichem IA product is the addition of three tumor markers to Multichem IA Plus; CA 125, CA 15-3 and CA 19-9. Multichem IA is designed as a tri-level, liquid stable frozen product. This product will typically be run after reagent calibration and at a frequency dictated by laboratory QC procedures and reagent / instrument manufacturer instructions. The product should be treated the same as patient specimens and run in accordance with the instructions accompanying the instrument, kit or reagent being used. The control should be stored at -20°C to –80°C and thawed as per IFU prior to use. The product should be stored at 2 to 8°C between use. -

 Multichem IA Plus Control is intended for use as a third party, multi-constituent quality control material to monitor the precision of laboratory testing procedures for Immunoassay Assays. Multichem IA Plus contains 86 analytes including fertility and thyroid hormones, steroid hormones, cardiac markers, anaemia markers, therapeutic drugs, adrenal markers, bone metabolism markers and tumour markers. Multichem IA Plus, from Technopath Manufacturing Ltd, is designed as a tri-level, liquid stable frozen product. This product will typically be run after reagent calibration and at a frequency dictated by laboratory QC procedures and reagent / instrument manufacturer instructions. The product should be treated the same as patient specimens and run in accordance with the instructions accompanying the instrument, kit or reagent being used. The control should be stored at -20°C to –80°C and thawed as per IFU prior to use. The product should be stored at 2 to 8°C between use.
Multichem IA Plus Control is intended for use as a third party, multi-constituent quality control material to monitor the precision of laboratory testing procedures for Immunoassay Assays. Multichem IA Plus contains 86 analytes including fertility and thyroid hormones, steroid hormones, cardiac markers, anaemia markers, therapeutic drugs, adrenal markers, bone metabolism markers and tumour markers. Multichem IA Plus, from Technopath Manufacturing Ltd, is designed as a tri-level, liquid stable frozen product. This product will typically be run after reagent calibration and at a frequency dictated by laboratory QC procedures and reagent / instrument manufacturer instructions. The product should be treated the same as patient specimens and run in accordance with the instructions accompanying the instrument, kit or reagent being used. The control should be stored at -20°C to –80°C and thawed as per IFU prior to use. The product should be stored at 2 to 8°C between use. -
 Multichem IA Speciality Immunoassay Controls are intended for use as third party, tri- level, liquid stable, multi-analyte quality control materials to monitor the precision of laboratory testing procedures for Immunoassay Assays. Multichem IA Speciality, from Technopath Manufacturing Ltd, is designed to complement Multichem IA and Multichem IA Plus Immunoassay Controls by offering significantly increased stability for BNP, PTH and ACTH. In addition to these analytes, Multichem IA Speciality Controls also contains and provides tri level utility for Procalcitonin and Calcitonin. Multichem IA Speciality Immunoassay Controls are designed as tri-level, liquid stable frozen products. These products will typically be run after reagent calibration and at a frequency dictated by laboratory QC procedures and reagent / instrument manufacturer instructions. The products should be treated the same as patient specimens and run in accordance with the instructions accompanying the instrument, kit or reagent being used. The controls should be stored at -20°C to –80°C and thawed as per IFU prior to use. The products should be stored at 2 to 8°C between use.
Multichem IA Speciality Immunoassay Controls are intended for use as third party, tri- level, liquid stable, multi-analyte quality control materials to monitor the precision of laboratory testing procedures for Immunoassay Assays. Multichem IA Speciality, from Technopath Manufacturing Ltd, is designed to complement Multichem IA and Multichem IA Plus Immunoassay Controls by offering significantly increased stability for BNP, PTH and ACTH. In addition to these analytes, Multichem IA Speciality Controls also contains and provides tri level utility for Procalcitonin and Calcitonin. Multichem IA Speciality Immunoassay Controls are designed as tri-level, liquid stable frozen products. These products will typically be run after reagent calibration and at a frequency dictated by laboratory QC procedures and reagent / instrument manufacturer instructions. The products should be treated the same as patient specimens and run in accordance with the instructions accompanying the instrument, kit or reagent being used. The controls should be stored at -20°C to –80°C and thawed as per IFU prior to use. The products should be stored at 2 to 8°C between use. -
 Third party, single elevated level, liquid stable quality control material to monitor the precision of laboratory testing procedures for Bilirubin and Theophylline Assays. Multichem NB (Neonatal Bilirubin) Control, from Technopath Manufacturing Ltd, is intended for use as a third party, single level, liquid stable quality control material to monitor the precision of laboratory testing procedures for Bilirubin and Theophylline Assays. An important patient population is Neonates, where a requirement has been identified for testing conditions associated with Neonatal hyperbilirubinemia. The product also contains Theophylline, a therapeutic drug that is given to Neonates to improve lung capacity and where it would be required to monitor for toxicity risk. Caffeine is a listed analyte that is added gravimetrically, and this has a clinical function as the primary treatment of the breathing disorders apnea of prematurity. Another important population is with any patient that has liver failure. It is important if the laboratory tests hyperbilirubinemia patient samples that they analyze QC regularly to monitor this range to ensure accuracy and precision at these elevated levels. The requirement for a high concentration of Bilirubin presents a conflict for consolidation of multi-analytes. This means that not only is a separate paediatric control required, but a control matrix that is treated with antioxidants to inhibit the oxidation of Bilirubin. The application of the most appropriate measures to reduce the effect of oxidation on the high levels of Bilirubin in the Control have been researched to ensure that open vial and closed stability is maintained. Multichem NB Control is designed as a single level, liquid stable frozen product. The product will typically be run as a supplementary high level control, in conjunction with at least one of the Multichem Serum Control levels, (S or S Plus) to provide a minimum of 2 levels of control to allow assay system performance monitoring within the analytical ranges. It will be run after reagent calibration and at a frequency dictated by laboratory QC procedures and reagent / instrument manufacturer instructions. The product should be treated the same as patient specimens and run in accordance with the instructions accompanying the instrument, kit or reagent being used. The control is to be stored at -20°C to –80°C and thawed as per IFU prior to use. The product is to be stored at 2 to 8°C between use.
Third party, single elevated level, liquid stable quality control material to monitor the precision of laboratory testing procedures for Bilirubin and Theophylline Assays. Multichem NB (Neonatal Bilirubin) Control, from Technopath Manufacturing Ltd, is intended for use as a third party, single level, liquid stable quality control material to monitor the precision of laboratory testing procedures for Bilirubin and Theophylline Assays. An important patient population is Neonates, where a requirement has been identified for testing conditions associated with Neonatal hyperbilirubinemia. The product also contains Theophylline, a therapeutic drug that is given to Neonates to improve lung capacity and where it would be required to monitor for toxicity risk. Caffeine is a listed analyte that is added gravimetrically, and this has a clinical function as the primary treatment of the breathing disorders apnea of prematurity. Another important population is with any patient that has liver failure. It is important if the laboratory tests hyperbilirubinemia patient samples that they analyze QC regularly to monitor this range to ensure accuracy and precision at these elevated levels. The requirement for a high concentration of Bilirubin presents a conflict for consolidation of multi-analytes. This means that not only is a separate paediatric control required, but a control matrix that is treated with antioxidants to inhibit the oxidation of Bilirubin. The application of the most appropriate measures to reduce the effect of oxidation on the high levels of Bilirubin in the Control have been researched to ensure that open vial and closed stability is maintained. Multichem NB Control is designed as a single level, liquid stable frozen product. The product will typically be run as a supplementary high level control, in conjunction with at least one of the Multichem Serum Control levels, (S or S Plus) to provide a minimum of 2 levels of control to allow assay system performance monitoring within the analytical ranges. It will be run after reagent calibration and at a frequency dictated by laboratory QC procedures and reagent / instrument manufacturer instructions. The product should be treated the same as patient specimens and run in accordance with the instructions accompanying the instrument, kit or reagent being used. The control is to be stored at -20°C to –80°C and thawed as per IFU prior to use. The product is to be stored at 2 to 8°C between use. -
 Multichem P (P for protein), from Technopath Manufacturing Ltd, is specifically designed as a supplementary QC product for immunoproteins to be used in conjunction with the Multichem clinical chemistry QC. This product offers elevated concentrations for immunoprotein. Users can use this in conjunction with 1, 2 or 3 levels of Multichem S Plus or Multichem S, each of which already include multiple concentrations for immunoproteins. The full list of products available are shown below. Multichem P Supplementary Immunoprotein Control is intended for use as a third party, single level, liquid stable immunoprotein quality control material to monitor the precision of laboratory testing procedures for Immunoprotein Assays. Target values are provided for 39 analytes, including immunoglobulins, complement proteins, inflammatory proteins as well as carrier and storage proteins. The product will typically be run as a supplementary high level control, in conjunction with at least one of the Multichem Serum Control levels, (S or S Plus) to provide a minimum of 2 levels of control to allow assay system performance monitoring within the analytical ranges. It will be run after reagent calibration and at a frequency dictated by laboratory QC procedures and reagent / instrument manufacturer instructions. The product should be treated the same as patient specimens and run in accordance with the instructions accompanying the instrument, kit or reagent being used. The control is to be stored at -20°C to –80°C and thawed as per IFU prior to use. The product is to be stored at 2 to 8°C between use.
Multichem P (P for protein), from Technopath Manufacturing Ltd, is specifically designed as a supplementary QC product for immunoproteins to be used in conjunction with the Multichem clinical chemistry QC. This product offers elevated concentrations for immunoprotein. Users can use this in conjunction with 1, 2 or 3 levels of Multichem S Plus or Multichem S, each of which already include multiple concentrations for immunoproteins. The full list of products available are shown below. Multichem P Supplementary Immunoprotein Control is intended for use as a third party, single level, liquid stable immunoprotein quality control material to monitor the precision of laboratory testing procedures for Immunoprotein Assays. Target values are provided for 39 analytes, including immunoglobulins, complement proteins, inflammatory proteins as well as carrier and storage proteins. The product will typically be run as a supplementary high level control, in conjunction with at least one of the Multichem Serum Control levels, (S or S Plus) to provide a minimum of 2 levels of control to allow assay system performance monitoring within the analytical ranges. It will be run after reagent calibration and at a frequency dictated by laboratory QC procedures and reagent / instrument manufacturer instructions. The product should be treated the same as patient specimens and run in accordance with the instructions accompanying the instrument, kit or reagent being used. The control is to be stored at -20°C to –80°C and thawed as per IFU prior to use. The product is to be stored at 2 to 8°C between use. -
 Multichem S Plus Control, from Technopath Manufacturing Ltd, is intended for use as a third party, multi-constituent quality control material to monitor the precision of laboratory testing procedures for Chemistry Assays. It incorporates serum chemistry, immunology, lipid, TDM, enzymes and esoterics. Multichem S Plus contains 105 analytes including the addition of C-Reactive Protein and Rheumatoid Factor at clinically relevant concentrations. Chemistry, Esoterics, Immunoproteins, Enzymes, Lipids, and Therapeutic Drugs are also included. When combined with Multichem P the solution offers an extensive spread of immunoprotein concentrations. Multichem S is also available, please note that Multichem S contains all the same analytes apart from C-Reactive Protein and Rhematoid Factor. Multichem S Plus is designed as a tri-level, liquid stable frozen product. This product will typically be run after reagent calibration and at a frequency dictated by laboratory QC procedures and reagent / instrument manufacturer instructions. The product should be treated the same as patient specimens and run in accordance with the instructions accompanying the instrument, kit or reagent being used. The control should be stored at -20°C to –80°C and thawed as per IFU prior to use. The product should be stored at 2 to 8°C between use.
Multichem S Plus Control, from Technopath Manufacturing Ltd, is intended for use as a third party, multi-constituent quality control material to monitor the precision of laboratory testing procedures for Chemistry Assays. It incorporates serum chemistry, immunology, lipid, TDM, enzymes and esoterics. Multichem S Plus contains 105 analytes including the addition of C-Reactive Protein and Rheumatoid Factor at clinically relevant concentrations. Chemistry, Esoterics, Immunoproteins, Enzymes, Lipids, and Therapeutic Drugs are also included. When combined with Multichem P the solution offers an extensive spread of immunoprotein concentrations. Multichem S is also available, please note that Multichem S contains all the same analytes apart from C-Reactive Protein and Rhematoid Factor. Multichem S Plus is designed as a tri-level, liquid stable frozen product. This product will typically be run after reagent calibration and at a frequency dictated by laboratory QC procedures and reagent / instrument manufacturer instructions. The product should be treated the same as patient specimens and run in accordance with the instructions accompanying the instrument, kit or reagent being used. The control should be stored at -20°C to –80°C and thawed as per IFU prior to use. The product should be stored at 2 to 8°C between use.

