-
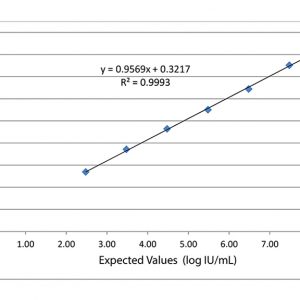
 Validate and Monitor your assays AccuSpan linearity panels from SeraCare are designed to span the dynamic range of quantitative infectious disease assays and evaluate instrumentation analytical sensitivity. Linearity panels effectively challenge assay performance at defined intervals to ensure consistency throughout the entire reportable range. In addition to linearity studies, these panels are useful in validation procedures for new assay implementation, operator training, and troubleshooting signs of assay deterioration.
Validate and Monitor your assays AccuSpan linearity panels from SeraCare are designed to span the dynamic range of quantitative infectious disease assays and evaluate instrumentation analytical sensitivity. Linearity panels effectively challenge assay performance at defined intervals to ensure consistency throughout the entire reportable range. In addition to linearity studies, these panels are useful in validation procedures for new assay implementation, operator training, and troubleshooting signs of assay deterioration. -

 Ensure reagents are operating effectively from lot-to-lot AccuTrak qualification panels from SeraCare are designed as a cost-effective solution to deliver the consistent results you need to gain confidence in your assay’s performance and ensure reagents are operating effectively from lot-to-lot. SeraCare's AccuTrak qualification panels are utilised by clinical laboratories worldwide to help strengthen quality control protocols and procedures for infectious disease diagnostic assays. With products for HIV, hepatitis, CMV, syphilis, HPV, and HTLV, SeraCare offers a comprehensive portfolio to help effectively monitor assay performance. Reliable, Consistent, Cost-Effective Solutions for Your Assay QC Program
Ensure reagents are operating effectively from lot-to-lot AccuTrak qualification panels from SeraCare are designed as a cost-effective solution to deliver the consistent results you need to gain confidence in your assay’s performance and ensure reagents are operating effectively from lot-to-lot. SeraCare's AccuTrak qualification panels are utilised by clinical laboratories worldwide to help strengthen quality control protocols and procedures for infectious disease diagnostic assays. With products for HIV, hepatitis, CMV, syphilis, HPV, and HTLV, SeraCare offers a comprehensive portfolio to help effectively monitor assay performance. Reliable, Consistent, Cost-Effective Solutions for Your Assay QC Program -

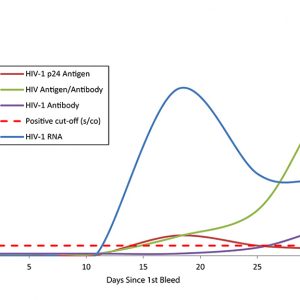 Assess your assay development milestones When your assay development requires natural patient specimens that represent the body’s true response to an infection, you can depend on AccuVert seroconversion panels as a gold standard with which to assess your assay development milestones. The SeraCare seroconversion panels are developed using raw, undiluted plasma collected from a single individual during the development of an infection and subsequent immunological response. Spanning an array of infectious diseases from HIV to hepatitis and syphilis, SeraCare's panels provide you with a diverse selection of products with high-quality datasets to help evaluate your assay. A Rich History in Seroconversion Panels SeraCare have been a trusted provider of seroconversion panels for over 30 years, IVD diagnostic manufacturers worldwide have used our panels in the development and validation of their assays for decades. As shown in the World Health Organisation HIV test evaluation kit reports, SeraCare HIV seroconversion panels have been used for comparative studies for HIV test kit evaluations, and are also frequently referenced in package inserts of leading IVD infectious disease platforms.
Assess your assay development milestones When your assay development requires natural patient specimens that represent the body’s true response to an infection, you can depend on AccuVert seroconversion panels as a gold standard with which to assess your assay development milestones. The SeraCare seroconversion panels are developed using raw, undiluted plasma collected from a single individual during the development of an infection and subsequent immunological response. Spanning an array of infectious diseases from HIV to hepatitis and syphilis, SeraCare's panels provide you with a diverse selection of products with high-quality datasets to help evaluate your assay. A Rich History in Seroconversion Panels SeraCare have been a trusted provider of seroconversion panels for over 30 years, IVD diagnostic manufacturers worldwide have used our panels in the development and validation of their assays for decades. As shown in the World Health Organisation HIV test evaluation kit reports, SeraCare HIV seroconversion panels have been used for comparative studies for HIV test kit evaluations, and are also frequently referenced in package inserts of leading IVD infectious disease platforms. -

 Accelerate assay optimisation, simplify regulatory compliance, and ensure quality. Understand and manage the performance of your automated liquid handlers, multichannel pipettes, labware, operators, and more. Compatible with virtually all automated liquid handling systems and multichannel handheld pipettes, the Artel MVS sits on a mobile workstation for portable rapid calibration, verifications and optimisation of dispensed volumes with high precision and accuracy. The MVS’s unique, dual-dye photometric measurements are robust against environmental influences and traceable to SI units to enable comparison across operators, protocols, equipment, and locations. Using the Calibrator Plate, Verification Plates, and an Artel-certified Plate Reader, the MVS supports an unbroken chain of traceability to national and international standards.
Accelerate assay optimisation, simplify regulatory compliance, and ensure quality. Understand and manage the performance of your automated liquid handlers, multichannel pipettes, labware, operators, and more. Compatible with virtually all automated liquid handling systems and multichannel handheld pipettes, the Artel MVS sits on a mobile workstation for portable rapid calibration, verifications and optimisation of dispensed volumes with high precision and accuracy. The MVS’s unique, dual-dye photometric measurements are robust against environmental influences and traceable to SI units to enable comparison across operators, protocols, equipment, and locations. Using the Calibrator Plate, Verification Plates, and an Artel-certified Plate Reader, the MVS supports an unbroken chain of traceability to national and international standards. -

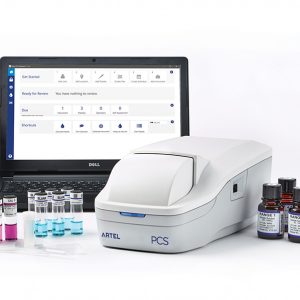 Ensure data integrity, reliable test results, and regulatory compliance. Easily calibrate pipettes, perform interim verifications, standardise operator skills, manage pipette inventory, and more with the Artel PCS® Pipette Calibration System. Fast, accurate, and precise, the PCS is a portable and easy-to-use volume verification system that simplifies single-channel pipette calibration, interim volume verification, and pipette user/operator training and competency assessment. Paired with the included PCS Software, the PCS helps you stay on top of your pipette inventory with calibration and interim verification scheduling, email notifications, pipette and pipette operator status, and comprehensive, auditable documentation. Easily meet both external regulatory requirements and internal quality standards to ensure data quality. The power of the PCS stems from the ratiometric photometry technology and standardised dye solutions that are used to measure dispensed volumes. The system is robust against differences in environmental conditions and calibration results are traceable to NIST standards, enabling straightforward comparison of pipettes, operators, methods, and locations.
Ensure data integrity, reliable test results, and regulatory compliance. Easily calibrate pipettes, perform interim verifications, standardise operator skills, manage pipette inventory, and more with the Artel PCS® Pipette Calibration System. Fast, accurate, and precise, the PCS is a portable and easy-to-use volume verification system that simplifies single-channel pipette calibration, interim volume verification, and pipette user/operator training and competency assessment. Paired with the included PCS Software, the PCS helps you stay on top of your pipette inventory with calibration and interim verification scheduling, email notifications, pipette and pipette operator status, and comprehensive, auditable documentation. Easily meet both external regulatory requirements and internal quality standards to ensure data quality. The power of the PCS stems from the ratiometric photometry technology and standardised dye solutions that are used to measure dispensed volumes. The system is robust against differences in environmental conditions and calibration results are traceable to NIST standards, enabling straightforward comparison of pipettes, operators, methods, and locations. -

 Internal Quality Control Software from Technopath Manufacturing Ltd. IAMQC Daily is a comprehensive Internal Quality Control software that applies Westgard and/or any user-defined QC rules to individual QC results. The software automatically builds interactive Levey-Jennings charts and tables and provides summary and detailed customised reports to the end user. IAMQC Daily integrates with Microsoft Excel to produce customised electronic reports. The system also allows the import of pre-defined templates, resulting in instant system setups. Users can create audit trails, action logs and summary reports at the click of a button. IAMQC Daily comprises a centralised program that facilitates the analysis of multiple QC materials, across numerous departments in a laboratory setting. The system can also be integrated with IAMQC Peer to satisfy both Internal and External QC requirements. IAMQC Daily offers a centralised review of all QC data from all laboratories/instruments. Central administrator access facilitates managers to review QC performance at multiple facilities – no need to visit each laboratory site. Closer monitoring of QC from remote locations without additional costs provides a flexible option for managing the inter and intra-lab performance. The software works on an ‘open’ platform that allows the end user to add all types of control material from a range of laboratories. Both IAMQC Daily and Expert can run using an internet OR intranet connection. Each PC license allows the user to manage an unlimited number of departments, control materials (not limited to Technopath materials) and instruments. An unlimited number of user logins can be added to the system at any stage. An administrator module can also manage user logins, customising the functionality that is available to each user. All data can also be filtered using the same logic to apply a user-friendly atmosphere and save time scrolling through data.
Internal Quality Control Software from Technopath Manufacturing Ltd. IAMQC Daily is a comprehensive Internal Quality Control software that applies Westgard and/or any user-defined QC rules to individual QC results. The software automatically builds interactive Levey-Jennings charts and tables and provides summary and detailed customised reports to the end user. IAMQC Daily integrates with Microsoft Excel to produce customised electronic reports. The system also allows the import of pre-defined templates, resulting in instant system setups. Users can create audit trails, action logs and summary reports at the click of a button. IAMQC Daily comprises a centralised program that facilitates the analysis of multiple QC materials, across numerous departments in a laboratory setting. The system can also be integrated with IAMQC Peer to satisfy both Internal and External QC requirements. IAMQC Daily offers a centralised review of all QC data from all laboratories/instruments. Central administrator access facilitates managers to review QC performance at multiple facilities – no need to visit each laboratory site. Closer monitoring of QC from remote locations without additional costs provides a flexible option for managing the inter and intra-lab performance. The software works on an ‘open’ platform that allows the end user to add all types of control material from a range of laboratories. Both IAMQC Daily and Expert can run using an internet OR intranet connection. Each PC license allows the user to manage an unlimited number of departments, control materials (not limited to Technopath materials) and instruments. An unlimited number of user logins can be added to the system at any stage. An administrator module can also manage user logins, customising the functionality that is available to each user. All data can also be filtered using the same logic to apply a user-friendly atmosphere and save time scrolling through data. -

 Select QC Rules and make meaningful QC decisions IAMQC Expert, from Technopath Manufacturing Ltd, is an interactive system that helps front line laboratory staff select QC rules, reduce unnecessary repeats and make meaningful QC decisions. IAMQC Expert allows the end-user to monitor method performance relative to clinical requirements and focus on the tests that require their attention. Times and technologies are changing rapidly. Instruments and methodologies are more accurate, precise and stable than they were a decade ago. Most laboratories have adopted these new technical advances, but few have modified their QC processes to match. Many laboratories are still using a 1-2s rule as recommended by Levey and Jennings in 1951. Technopath Manufacturing Ltd have designed a QC system that will alert users to significant changes and not generate QC flags when the system is operating safely within acceptable limits. The system compares method performance to defined quality requirements (rather than to last month’s data) and recommends QC strategies that will warn users when QC data points exceed acceptable performance - with a minimal number of false flags. In the design of our QC system we “balance” the quality control system to meet the changing performance and stability of the analytical system.
Select QC Rules and make meaningful QC decisions IAMQC Expert, from Technopath Manufacturing Ltd, is an interactive system that helps front line laboratory staff select QC rules, reduce unnecessary repeats and make meaningful QC decisions. IAMQC Expert allows the end-user to monitor method performance relative to clinical requirements and focus on the tests that require their attention. Times and technologies are changing rapidly. Instruments and methodologies are more accurate, precise and stable than they were a decade ago. Most laboratories have adopted these new technical advances, but few have modified their QC processes to match. Many laboratories are still using a 1-2s rule as recommended by Levey and Jennings in 1951. Technopath Manufacturing Ltd have designed a QC system that will alert users to significant changes and not generate QC flags when the system is operating safely within acceptable limits. The system compares method performance to defined quality requirements (rather than to last month’s data) and recommends QC strategies that will warn users when QC data points exceed acceptable performance - with a minimal number of false flags. In the design of our QC system we “balance” the quality control system to meet the changing performance and stability of the analytical system. -

 Innovative, real-time, Peer Comparison Software from Technopath Manufacturing Ltd. The web based system facilitates laboratories testing the same lot number of control material to access valuable information from their colleagues through peer comparison. The reports that are generated in IAMQC Peer compare the accuracy and precision of analytical processes between laboratories and peer groups. This information can be extremely valuable, indicating the user’s performance relative to their peer group and also providing powerful troubleshooting tools when attempting to resolve potential problems. To participate in IAMQC Peer, each individual laboratory submits their individual results or summary statistics (mean, standard deviation, and number of data points) to the central database maintained by Technopath Manufacturing Ltd. Laboratories data may be submitted manually on-line or, alternatively, captured by one of our many interfacing options. The information provided by IAMQC Peer can be used on a monthly basis to evaluate how well lab’s methods are operating, relative to the overall peer group. Users can also look at this peer data in real-time, interactive, tables online, when they are investigating a potential problem with accuracy or precision for an individual method.
Innovative, real-time, Peer Comparison Software from Technopath Manufacturing Ltd. The web based system facilitates laboratories testing the same lot number of control material to access valuable information from their colleagues through peer comparison. The reports that are generated in IAMQC Peer compare the accuracy and precision of analytical processes between laboratories and peer groups. This information can be extremely valuable, indicating the user’s performance relative to their peer group and also providing powerful troubleshooting tools when attempting to resolve potential problems. To participate in IAMQC Peer, each individual laboratory submits their individual results or summary statistics (mean, standard deviation, and number of data points) to the central database maintained by Technopath Manufacturing Ltd. Laboratories data may be submitted manually on-line or, alternatively, captured by one of our many interfacing options. The information provided by IAMQC Peer can be used on a monthly basis to evaluate how well lab’s methods are operating, relative to the overall peer group. Users can also look at this peer data in real-time, interactive, tables online, when they are investigating a potential problem with accuracy or precision for an individual method. -

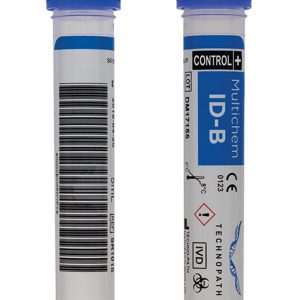 Positive multi-marker QC Multichem Infectious Disease (ID) Quality Controls (QC) samples, have been specifically optimised and validated to match assays commonly used for infectious diseases testing. Multichem ID-B is an unassayed control, optimised for Abbott and Diasorin instruments. Scroll down to view the product benefits and analyte list. The Multichem ID Multi-marker range of products is easily identifiable by their colour coding (in this case B for blue). Multichem ID-B consolidates six analytes (see analyte list below). This unassayed product is optimised for specific platforms, including; Abbott Architect, Abbott Alinity i, Abbott Alinity s, DiaSorin Liaison, DiaSorin Liaison XL MUREX.
Positive multi-marker QC Multichem Infectious Disease (ID) Quality Controls (QC) samples, have been specifically optimised and validated to match assays commonly used for infectious diseases testing. Multichem ID-B is an unassayed control, optimised for Abbott and Diasorin instruments. Scroll down to view the product benefits and analyte list. The Multichem ID Multi-marker range of products is easily identifiable by their colour coding (in this case B for blue). Multichem ID-B consolidates six analytes (see analyte list below). This unassayed product is optimised for specific platforms, including; Abbott Architect, Abbott Alinity i, Abbott Alinity s, DiaSorin Liaison, DiaSorin Liaison XL MUREX. -

 Positive quality control for antibodies to SARS-CoV-2 (including IgG) Multichem ID-COVID19 G is an unasssayed third-party quality control for Antibodies to SARS-CoV-2 (including IgG). This Quality Control is available with access to the Technopath Clinical Diagnostics inter-laboratory Peer program, IAMQC®. The Multichem ID-COVID19 G (unassayed) is a positive control for antibodies to the SARS-CoV-2 virus (including IgG). The kit is delivered in a choice of two configurations: 4x2mL tubes with an integrated instrument barcode and secondly 4 x 4mL vials. The product is suitable for use on all major platforms, including amongst others, Abbott, Roche and Siemens. All Multichem ID-COVID19 quality controls are supported by the inter-laboratory Peer program - IAMQC®.
Positive quality control for antibodies to SARS-CoV-2 (including IgG) Multichem ID-COVID19 G is an unasssayed third-party quality control for Antibodies to SARS-CoV-2 (including IgG). This Quality Control is available with access to the Technopath Clinical Diagnostics inter-laboratory Peer program, IAMQC®. The Multichem ID-COVID19 G (unassayed) is a positive control for antibodies to the SARS-CoV-2 virus (including IgG). The kit is delivered in a choice of two configurations: 4x2mL tubes with an integrated instrument barcode and secondly 4 x 4mL vials. The product is suitable for use on all major platforms, including amongst others, Abbott, Roche and Siemens. All Multichem ID-COVID19 quality controls are supported by the inter-laboratory Peer program - IAMQC®. -

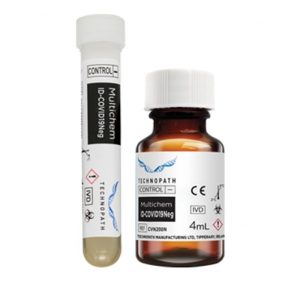 Negative quality control for antibodies to SARS-CoV-2 (including IgG) Multichem ID-COVID19Neg is an unassayed negative third-party quality control for antibodies to SARS-CoV-2 (including IgG). This Quality Control is available with access to the Technopath Clinical Diagnostics inter-laboratory Peer program, IAMQC®. The Multichem ID-COVID19Neg (unassayed) is an unassayed negative third-party quality control for antibodies to SARS-CoV-2 (including IgG). The kit is delivered in a choice of two configurations: 4x2mL tubes with an integrated instrument barcode and secondly 4 x 4mL vials. The product is suitable for use on all major platforms, including amongst others, Abbott, Roche and Siemens. All Multichem ID-COVID19 quality controls are supported by the inter-laboratory Peer program - IAMQC®.
Negative quality control for antibodies to SARS-CoV-2 (including IgG) Multichem ID-COVID19Neg is an unassayed negative third-party quality control for antibodies to SARS-CoV-2 (including IgG). This Quality Control is available with access to the Technopath Clinical Diagnostics inter-laboratory Peer program, IAMQC®. The Multichem ID-COVID19Neg (unassayed) is an unassayed negative third-party quality control for antibodies to SARS-CoV-2 (including IgG). The kit is delivered in a choice of two configurations: 4x2mL tubes with an integrated instrument barcode and secondly 4 x 4mL vials. The product is suitable for use on all major platforms, including amongst others, Abbott, Roche and Siemens. All Multichem ID-COVID19 quality controls are supported by the inter-laboratory Peer program - IAMQC®. -

 Negative multi-marker QC Multichem Infectious Disease (ID) Quality Controls (QC) samples, have been specifically optimised and validated to match assays commonly used for infectious diseases testing. Multichem ID-SeroNeg provides a negative control for the analytes listed below. Scroll down to view the product benefits and analyte list. Multichem ID SeroNeg provides a third party, unassayed, negative control for commonly tested assays for anti-HIV, anti-HCV, anti HTLV, anti-HBc, anti-Treponema, HBsAg and HIVp24
Negative multi-marker QC Multichem Infectious Disease (ID) Quality Controls (QC) samples, have been specifically optimised and validated to match assays commonly used for infectious diseases testing. Multichem ID-SeroNeg provides a negative control for the analytes listed below. Scroll down to view the product benefits and analyte list. Multichem ID SeroNeg provides a third party, unassayed, negative control for commonly tested assays for anti-HIV, anti-HCV, anti HTLV, anti-HBc, anti-Treponema, HBsAg and HIVp24

