- Non-infectious and replication deficient, enables safe handling of positive material
- Fully-extractable with a real viral protein coat; serves as a full-process reference material
- Compatible with assays targeting CDC and WHO consensus sequences
- Includes negative reference material for targeting sequences for the human RNAse P gene
- 2 year stability at 2 - 8°C
- Customisable to sequences of interest to meet unique assay design requirements
-

 Full Genome Coverage now available AccuPlex™ SARS-CoV-2 Verification Panel, from SeraCare, is optimised for assay verification at installation by documenting test performance along the assay’s range enabling laboratories to establish lower limits of detection, perform assay comparisons, and evaluate staff proficiency. The product contains positive materials including the full SARS-CoV-2 viral genome, and negative materials targeting the human RNase P gene. AccuPlex™ SARS-CoV-2 Verification Panel, coupled with the AccuPlex™ SARS-CoV-2 Reference Material, is a complete Quality Solution designed to challenge the entire molecular test procedure ensuring clinical laboratories can have confidence in their SARS-CoV-2 assay results.
Full Genome Coverage now available AccuPlex™ SARS-CoV-2 Verification Panel, from SeraCare, is optimised for assay verification at installation by documenting test performance along the assay’s range enabling laboratories to establish lower limits of detection, perform assay comparisons, and evaluate staff proficiency. The product contains positive materials including the full SARS-CoV-2 viral genome, and negative materials targeting the human RNase P gene. AccuPlex™ SARS-CoV-2 Verification Panel, coupled with the AccuPlex™ SARS-CoV-2 Reference Material, is a complete Quality Solution designed to challenge the entire molecular test procedure ensuring clinical laboratories can have confidence in their SARS-CoV-2 assay results. -

 Have confidence in your multiplex assay results for SARS-CoV-2 & Flu A/B AccuPlex™ SARS-CoV-2, Flu A/B and RSV Verification Panel, from SeraCare, is optimised for assay verification at installation by documenting test performance along the assay’s range, enabling laboratories to establish lower limits of detection, perform assay comparisons, and evaluate staff proficiency. Designed for use with multiplexed molecular assays that can detect SARS-CoV-2, influenza A/B and respiratory syncytial virus (RSV). AccuPlex™ SARS-CoV-2, Flu A/B and RSV Verification Panel, coupled with the AccuPlex™ SARS-CoV-2, Flu A/B and RSV Reference Material Kit, is a complete Quality Solution designed to challenge the entire molecular test procedure ensuring clinical laboratories can have confidence in their multiplex assay results.
Have confidence in your multiplex assay results for SARS-CoV-2 & Flu A/B AccuPlex™ SARS-CoV-2, Flu A/B and RSV Verification Panel, from SeraCare, is optimised for assay verification at installation by documenting test performance along the assay’s range, enabling laboratories to establish lower limits of detection, perform assay comparisons, and evaluate staff proficiency. Designed for use with multiplexed molecular assays that can detect SARS-CoV-2, influenza A/B and respiratory syncytial virus (RSV). AccuPlex™ SARS-CoV-2, Flu A/B and RSV Verification Panel, coupled with the AccuPlex™ SARS-CoV-2, Flu A/B and RSV Reference Material Kit, is a complete Quality Solution designed to challenge the entire molecular test procedure ensuring clinical laboratories can have confidence in their multiplex assay results.- Non-infectious and replication deficient, enables safe handling of positive material
- Fully-extractable with a real viral protein coat; serves as a full-process reference material
- 2 year stability at 2 - 8°C
- Optimised for assay verification and day-to-day performance monitoring
-

 Available for use with serological and molecular assays, SeraCare’s portfolio of AccuSet performance panels contains highly characterized, raw, undiluted plasma specimens collected from unique individuals positive for your analytes of interest. Each panel contains a comprehensive comparative data sheet with test results from a wide variety of leading commercially available assays and platforms. AccuSet performance panels can be used to evaluate assay specificity, sensitivity, repeatability, and reproducibility to assist you in validating new test methods and equipment, run head-to-head assay comparisons, demonstrate lab proficiency, and train laboratory personnel.
Available for use with serological and molecular assays, SeraCare’s portfolio of AccuSet performance panels contains highly characterized, raw, undiluted plasma specimens collected from unique individuals positive for your analytes of interest. Each panel contains a comprehensive comparative data sheet with test results from a wide variety of leading commercially available assays and platforms. AccuSet performance panels can be used to evaluate assay specificity, sensitivity, repeatability, and reproducibility to assist you in validating new test methods and equipment, run head-to-head assay comparisons, demonstrate lab proficiency, and train laboratory personnel. -

 Ensure antibody testing accuracy and performance AccuSet™ SARS-CoV-2 Performance Panel from SeraCare - This eleven-member validation panel consists of undiluted, naturally occurring human-plasma members. Ten panel members represent collections from multiple individuals positive for antibodies to SARS-CoV-2; a single negative member is also included. Test results from commercially available SARS-CoV-2 antibody assays are included for comparative analysis. AccuSet™ SARS-CoV-2 Performance Panel is intended to provide an out-of-the-box solution to evaluate SARS-CoV-2 antibody detection assays with highly characterized human specimens whether generating validation data for a regulatory submission or performing assay verification in a clinical laboratory setting. ACCURUN® Anti-SARS-CoV-2 Reference Material Kit is designed to support assay installation and monitoring of day-to-day assay performance, providing a complete quality solution for SARS-CoV-2 antibody testing.
Ensure antibody testing accuracy and performance AccuSet™ SARS-CoV-2 Performance Panel from SeraCare - This eleven-member validation panel consists of undiluted, naturally occurring human-plasma members. Ten panel members represent collections from multiple individuals positive for antibodies to SARS-CoV-2; a single negative member is also included. Test results from commercially available SARS-CoV-2 antibody assays are included for comparative analysis. AccuSet™ SARS-CoV-2 Performance Panel is intended to provide an out-of-the-box solution to evaluate SARS-CoV-2 antibody detection assays with highly characterized human specimens whether generating validation data for a regulatory submission or performing assay verification in a clinical laboratory setting. ACCURUN® Anti-SARS-CoV-2 Reference Material Kit is designed to support assay installation and monitoring of day-to-day assay performance, providing a complete quality solution for SARS-CoV-2 antibody testing. -
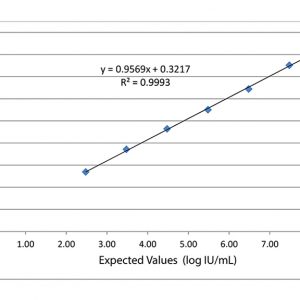
 Validate and Monitor your assays AccuSpan linearity panels from SeraCare are designed to span the dynamic range of quantitative infectious disease assays and evaluate instrumentation analytical sensitivity. Linearity panels effectively challenge assay performance at defined intervals to ensure consistency throughout the entire reportable range. In addition to linearity studies, these panels are useful in validation procedures for new assay implementation, operator training, and troubleshooting signs of assay deterioration.
Validate and Monitor your assays AccuSpan linearity panels from SeraCare are designed to span the dynamic range of quantitative infectious disease assays and evaluate instrumentation analytical sensitivity. Linearity panels effectively challenge assay performance at defined intervals to ensure consistency throughout the entire reportable range. In addition to linearity studies, these panels are useful in validation procedures for new assay implementation, operator training, and troubleshooting signs of assay deterioration. -

 Ensure reagents are operating effectively from lot-to-lot AccuTrak qualification panels from SeraCare are designed as a cost-effective solution to deliver the consistent results you need to gain confidence in your assay’s performance and ensure reagents are operating effectively from lot-to-lot. SeraCare's AccuTrak qualification panels are utilised by clinical laboratories worldwide to help strengthen quality control protocols and procedures for infectious disease diagnostic assays. With products for HIV, hepatitis, CMV, syphilis, HPV, and HTLV, SeraCare offers a comprehensive portfolio to help effectively monitor assay performance. Reliable, Consistent, Cost-Effective Solutions for Your Assay QC Program
Ensure reagents are operating effectively from lot-to-lot AccuTrak qualification panels from SeraCare are designed as a cost-effective solution to deliver the consistent results you need to gain confidence in your assay’s performance and ensure reagents are operating effectively from lot-to-lot. SeraCare's AccuTrak qualification panels are utilised by clinical laboratories worldwide to help strengthen quality control protocols and procedures for infectious disease diagnostic assays. With products for HIV, hepatitis, CMV, syphilis, HPV, and HTLV, SeraCare offers a comprehensive portfolio to help effectively monitor assay performance. Reliable, Consistent, Cost-Effective Solutions for Your Assay QC Program -

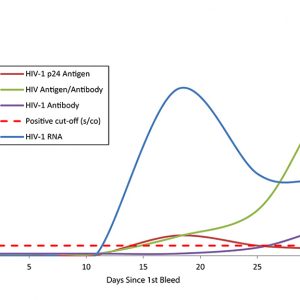 Assess your assay development milestones When your assay development requires natural patient specimens that represent the body’s true response to an infection, you can depend on AccuVert seroconversion panels as a gold standard with which to assess your assay development milestones. The SeraCare seroconversion panels are developed using raw, undiluted plasma collected from a single individual during the development of an infection and subsequent immunological response. Spanning an array of infectious diseases from HIV to hepatitis and syphilis, SeraCare's panels provide you with a diverse selection of products with high-quality datasets to help evaluate your assay. A Rich History in Seroconversion Panels SeraCare have been a trusted provider of seroconversion panels for over 30 years, IVD diagnostic manufacturers worldwide have used our panels in the development and validation of their assays for decades. As shown in the World Health Organisation HIV test evaluation kit reports, SeraCare HIV seroconversion panels have been used for comparative studies for HIV test kit evaluations, and are also frequently referenced in package inserts of leading IVD infectious disease platforms.
Assess your assay development milestones When your assay development requires natural patient specimens that represent the body’s true response to an infection, you can depend on AccuVert seroconversion panels as a gold standard with which to assess your assay development milestones. The SeraCare seroconversion panels are developed using raw, undiluted plasma collected from a single individual during the development of an infection and subsequent immunological response. Spanning an array of infectious diseases from HIV to hepatitis and syphilis, SeraCare's panels provide you with a diverse selection of products with high-quality datasets to help evaluate your assay. A Rich History in Seroconversion Panels SeraCare have been a trusted provider of seroconversion panels for over 30 years, IVD diagnostic manufacturers worldwide have used our panels in the development and validation of their assays for decades. As shown in the World Health Organisation HIV test evaluation kit reports, SeraCare HIV seroconversion panels have been used for comparative studies for HIV test kit evaluations, and are also frequently referenced in package inserts of leading IVD infectious disease platforms.

