-

 Accelerate assay optimisation, simplify regulatory compliance, and ensure quality. Understand and manage the performance of your automated liquid handlers, multichannel pipettes, labware, operators, and more. Compatible with virtually all automated liquid handling systems and multichannel handheld pipettes, the Artel MVS sits on a mobile workstation for portable rapid calibration, verifications and optimization of dispensed volumes with high precision and accuracy. The MVS’s unique, dual-dye photometric measurements are robust against environmental influences and traceable to SI units to enable comparison across operators, protocols, equipment, and locations. Using the Calibrator Plate, Verification Plates, and an Artel-certified Plate Reader, the MVS supports an unbroken chain of traceability to national and international standards.
Accelerate assay optimisation, simplify regulatory compliance, and ensure quality. Understand and manage the performance of your automated liquid handlers, multichannel pipettes, labware, operators, and more. Compatible with virtually all automated liquid handling systems and multichannel handheld pipettes, the Artel MVS sits on a mobile workstation for portable rapid calibration, verifications and optimization of dispensed volumes with high precision and accuracy. The MVS’s unique, dual-dye photometric measurements are robust against environmental influences and traceable to SI units to enable comparison across operators, protocols, equipment, and locations. Using the Calibrator Plate, Verification Plates, and an Artel-certified Plate Reader, the MVS supports an unbroken chain of traceability to national and international standards. -

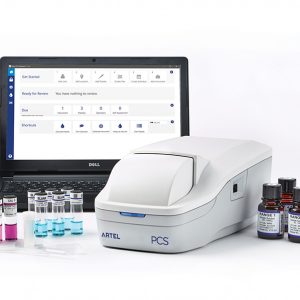 Ensure data integrity, reliable test results, and regulatory compliance. Easily calibrate pipettes, perform interim verifications, standardise operator skills, manage pipette inventory, and more with the Artel PCS® Pipette Calibration System. Fast, accurate, and precise, the PCS is a portable and easy-to-use volume verification system that simplifies single-channel pipette calibration, interim volume verification, and pipette user/operator training and competency assessment. Paired with the included PCS Software, the PCS helps you stay on top of your pipette inventory with calibration and interim verification scheduling, email notifications, pipette and pipette operator status, and comprehensive, auditable documentation. Easily meet both external regulatory requirements and internal quality standards to ensure data quality. The power of the PCS stems from the ratiometric photometry technology and standardised dye solutions that are used to measure dispensed volumes. The system is robust against differences in environmental conditions and calibration results are traceable to NIST standards, enabling straightforward comparison of pipettes, operators, methods, and locations.
Ensure data integrity, reliable test results, and regulatory compliance. Easily calibrate pipettes, perform interim verifications, standardise operator skills, manage pipette inventory, and more with the Artel PCS® Pipette Calibration System. Fast, accurate, and precise, the PCS is a portable and easy-to-use volume verification system that simplifies single-channel pipette calibration, interim volume verification, and pipette user/operator training and competency assessment. Paired with the included PCS Software, the PCS helps you stay on top of your pipette inventory with calibration and interim verification scheduling, email notifications, pipette and pipette operator status, and comprehensive, auditable documentation. Easily meet both external regulatory requirements and internal quality standards to ensure data quality. The power of the PCS stems from the ratiometric photometry technology and standardised dye solutions that are used to measure dispensed volumes. The system is robust against differences in environmental conditions and calibration results are traceable to NIST standards, enabling straightforward comparison of pipettes, operators, methods, and locations. -

 Introducing EMMA from Microtechnix EMMA is developed to digitise your Pharmaceutical Microbiology QC workflow. The (patent-pending) optical system captures 100% representative images for downstream analysis assisted by vision AI. This unique optical acquisition system allows to digitize your documentation process to ensure full data integrity. EMMA is a fully integrated system: including camera, built-in barcode reader and operating system.
Introducing EMMA from Microtechnix EMMA is developed to digitise your Pharmaceutical Microbiology QC workflow. The (patent-pending) optical system captures 100% representative images for downstream analysis assisted by vision AI. This unique optical acquisition system allows to digitize your documentation process to ensure full data integrity. EMMA is a fully integrated system: including camera, built-in barcode reader and operating system. -

 Introducing EMMA HT from Microtechnix EMMA HT can standardise and fully automate your Microbiological Environmental Monitoring. The (patent-pending) optics system captures ultra-precise images and uses artificial intelligence to analyse your samples. Perfect for environmental monitoring assays. This unique optical acquisition system allows to acquire high resolution image of 100% of the plate surface, including the wall (meniscus) of the Petri dish, and standardise your documentation process to ensure 100% data integrity. EMMA HT is a fully integrated system: including 2 EMMA devices, barcode reader, computer and the robot.
Introducing EMMA HT from Microtechnix EMMA HT can standardise and fully automate your Microbiological Environmental Monitoring. The (patent-pending) optics system captures ultra-precise images and uses artificial intelligence to analyse your samples. Perfect for environmental monitoring assays. This unique optical acquisition system allows to acquire high resolution image of 100% of the plate surface, including the wall (meniscus) of the Petri dish, and standardise your documentation process to ensure 100% data integrity. EMMA HT is a fully integrated system: including 2 EMMA devices, barcode reader, computer and the robot. -

 Introducing EMMA RL from Microtechnix EMMA RL provides a comprehensive solution to digitise & automate your pharmaceutical microbiology QC. The (patent-pending) optical system captures ultra-precise images and uses vision AI to analyse your data. This unique optical system allows to acquire 100% of the Petri dish content, including the wall & meniscus of the Petri dish. This allows the detection of any colony on the Petri Dishes, ensuring zero false negatives. This solution is fully integrated and automated.
Introducing EMMA RL from Microtechnix EMMA RL provides a comprehensive solution to digitise & automate your pharmaceutical microbiology QC. The (patent-pending) optical system captures ultra-precise images and uses vision AI to analyse your data. This unique optical system allows to acquire 100% of the Petri dish content, including the wall & meniscus of the Petri dish. This allows the detection of any colony on the Petri Dishes, ensuring zero false negatives. This solution is fully integrated and automated. -

 Microbial-coated test surfaces for evaluating surface sampling competency Qualification of personnel who collect surface samples is a regulatory requirement and a crucial activity for an effective environmental monitoring program. Personnel sampling performance, including percent recovery, consistency, and aseptic technique, drives the quality of data that is generated and significantly impacts the integrity of an environmental monitoring program. Introducing Enverify™, the industry standard for evaluating surface sampling competency. Enverify™ Test Surfaces, from Stratix Labs, are the first standardised tool that enables you to evaluate and document surface sampling competency with quantitative data. Each Enverify™ Test Surface contains a precise quantity of microorganisms, providing a benchmark for you to evaluate, verify, and compare performance. Enverify™ is designed for use with contact plates and swab sampling tools.
Microbial-coated test surfaces for evaluating surface sampling competency Qualification of personnel who collect surface samples is a regulatory requirement and a crucial activity for an effective environmental monitoring program. Personnel sampling performance, including percent recovery, consistency, and aseptic technique, drives the quality of data that is generated and significantly impacts the integrity of an environmental monitoring program. Introducing Enverify™, the industry standard for evaluating surface sampling competency. Enverify™ Test Surfaces, from Stratix Labs, are the first standardised tool that enables you to evaluate and document surface sampling competency with quantitative data. Each Enverify™ Test Surface contains a precise quantity of microorganisms, providing a benchmark for you to evaluate, verify, and compare performance. Enverify™ is designed for use with contact plates and swab sampling tools. -

 Test gram negative and gram positive bacteria in the same test panel Rapidly and accurately identify over 2,900 species of aerobic and anaerobic Bacteria, Yeasts, and Fungi using the Biolog Gen III Identification Phenotypic technology. Biolog’s advanced phenotypic technology provides valuable information on the properties of strains, in addition to a species-level identification. Molecular methods such as 16s sequencing and MALDI-TOF provide no information about the properties of the strain. Biolog’s carbon source utilisation technology identifies environmental and pathogenic microorganisms by producing a characteristic pattern or “metabolic fingerprint” from discrete test reactions performed within a 96 well microplate. Culture suspensions are tested with a panel of pre-selected assays, then incubated, read and compared to our extensive databases.
Test gram negative and gram positive bacteria in the same test panel Rapidly and accurately identify over 2,900 species of aerobic and anaerobic Bacteria, Yeasts, and Fungi using the Biolog Gen III Identification Phenotypic technology. Biolog’s advanced phenotypic technology provides valuable information on the properties of strains, in addition to a species-level identification. Molecular methods such as 16s sequencing and MALDI-TOF provide no information about the properties of the strain. Biolog’s carbon source utilisation technology identifies environmental and pathogenic microorganisms by producing a characteristic pattern or “metabolic fingerprint” from discrete test reactions performed within a 96 well microplate. Culture suspensions are tested with a panel of pre-selected assays, then incubated, read and compared to our extensive databases.- No Gram Stain
- No pre-tests
- No follow-on tests
- One panel for both Gram Negative & Gram Positive bacteria
- One minute set-up
- Over 1350 species coverage
-

 Microbial Controls made from your Environmental Isolates Tracking and trending environmental microbial isolates is a growing concern for pharmaceutical, nutraceutical, medical device, personal care product and food manufacturing laboratories. Microbiologics offers a comprehensive program for environmental isolate management. Our team of experts will identify, characterise, preserve and manufacture your strain into a test-ready control format for ongoing QC. Partner with TECHNOPATH and Microbiologics to reduce cost, minimize risk and increase confidence in your environmental monitoring program. Applications & Test Methods
Microbial Controls made from your Environmental Isolates Tracking and trending environmental microbial isolates is a growing concern for pharmaceutical, nutraceutical, medical device, personal care product and food manufacturing laboratories. Microbiologics offers a comprehensive program for environmental isolate management. Our team of experts will identify, characterise, preserve and manufacture your strain into a test-ready control format for ongoing QC. Partner with TECHNOPATH and Microbiologics to reduce cost, minimize risk and increase confidence in your environmental monitoring program. Applications & Test Methods- Antimicrobial Effectiveness Testing - USP <51>
- Aseptic Processing Environment - USP <1116>
- Disinfectant Qualification - USP <116>
- Growth Promotion Testing - USP <61>, <62>, <71>
- Suitability Testing - USP <51>, <61>, <62>, <71>
- Validation of Neutralisation Methods - USP <1227>
- Water for Pharmaceutical Purposes - USP <1231>
-

 Growth Promotion Testing EZ-Accu Shot, from Microbiologics®, provides a fast, convenient and reliable solution for Growth Promotion Testing, Media Challenge Testing and more. Each instant-dissolve microorganism pellet is designed to deliver 10-100 CFU per inoculum and provides 8 hours of stability after rehydration, so you can meet Pharmacopeia guidelines with ease. Applications & Test Methods
Growth Promotion Testing EZ-Accu Shot, from Microbiologics®, provides a fast, convenient and reliable solution for Growth Promotion Testing, Media Challenge Testing and more. Each instant-dissolve microorganism pellet is designed to deliver 10-100 CFU per inoculum and provides 8 hours of stability after rehydration, so you can meet Pharmacopeia guidelines with ease. Applications & Test Methods- Growth Promotion Testing
- Media Challenge Testing
- Suitability of Counting Methods
- Suitability of Sterility Tests
- Suitability of Tests for Specified Microorganisms
- Microbial Limits Testing
- Microbial Enumeration Testing
- Validation of Neutralization Methods
- Methods requiring a low CFU concentration
-

 Five compendial GPT strains, plus E. coli – all in one convenient kit EZ-Accu Shot Select, from Microbiologics®, contains the 5 compendial organisms for Growth Promotion Testing, plus E. coli for a total of 6 strains in one convenient kit. Perfect for qualifying EZ-Accu Shot for use in your laboratory, the EZ-Accu Shot Select kit contains one instant dissolve pellet of each of the 6 strains and 6 vials of hydration fluid. Each pellet delivers 10-100 CFU per inoculum with no serial dilutions and provided 8 hours of stability after rehydration for added flexibility to perform tests. EZ-Accu Shot Select strains are just 3 passages from the reference culture to meet Pharmacopeia standards. Strains Include:
Five compendial GPT strains, plus E. coli – all in one convenient kit EZ-Accu Shot Select, from Microbiologics®, contains the 5 compendial organisms for Growth Promotion Testing, plus E. coli for a total of 6 strains in one convenient kit. Perfect for qualifying EZ-Accu Shot for use in your laboratory, the EZ-Accu Shot Select kit contains one instant dissolve pellet of each of the 6 strains and 6 vials of hydration fluid. Each pellet delivers 10-100 CFU per inoculum with no serial dilutions and provided 8 hours of stability after rehydration for added flexibility to perform tests. EZ-Accu Shot Select strains are just 3 passages from the reference culture to meet Pharmacopeia standards. Strains Include:- Staphylococcus aureus subsp. aureus derived from ATCC® 6538™*
- Pseudomonas aeruginosa derived from ATCC® 9027™*
- Bacillus subtilis subsp. spizizenii derived from ATCC® 6633™*
- Candida albicans derived from ATCC® 10231™*
- Aspergillus brasiliensis derived from ATCC® 16404™*
- Escherichia coli derived from ATCC® 8739™*
-

 Economical Quantitative QC Microorganisms for Growth Promotion Testing EZ-CFU, from Microbiologics®, ready-to-use reference cultures for Growth Promotion Testing. Delivering 10-100 CFU per 0.1 ml inoculum, following a simple 1:10 dilution step, each microorganism suspension provides up to 90 tests. These handy kits allow for fast, reliable Growth Promotion Testing. Plus, EZ-CFU microorganisms are just three passages from the reference strains. All in all, EZ-CFU makes meeting Pharmacopeia standards for Growth Promotion Testing easier than ever. Applications & Test Methods
Economical Quantitative QC Microorganisms for Growth Promotion Testing EZ-CFU, from Microbiologics®, ready-to-use reference cultures for Growth Promotion Testing. Delivering 10-100 CFU per 0.1 ml inoculum, following a simple 1:10 dilution step, each microorganism suspension provides up to 90 tests. These handy kits allow for fast, reliable Growth Promotion Testing. Plus, EZ-CFU microorganisms are just three passages from the reference strains. All in all, EZ-CFU makes meeting Pharmacopeia standards for Growth Promotion Testing easier than ever. Applications & Test Methods- Growth Promotion Testing
- Media Challenge Testing
- Suitability of Counting Methods
- Suitability of Sterility Tests
- Suitability of Tests for Specified Microorganisms
- Microbial Limits Testing
- Microbial Enumeration Testing
- Validation of Neutralisation Methods
- Methods requiring a low CFU concentration
-

 Ready-to-Use Quantitative QC Microorganisms for Growth Promotion Testing Introducing EZ-CFU One Step, from Microbiologics®. This product is designed specifically for Growth Promotion Testing, Suitability of Tests for Specified Microorganisms, Suitability of Counting Methods and more. These ready-to-use quantitative microorganisms are just 3 passages from the reference culture and deliver 10-100 CFU per inoculum, as required by the Pharmacopeias, with minimal prep time. Applications & Test Methods
Ready-to-Use Quantitative QC Microorganisms for Growth Promotion Testing Introducing EZ-CFU One Step, from Microbiologics®. This product is designed specifically for Growth Promotion Testing, Suitability of Tests for Specified Microorganisms, Suitability of Counting Methods and more. These ready-to-use quantitative microorganisms are just 3 passages from the reference culture and deliver 10-100 CFU per inoculum, as required by the Pharmacopeias, with minimal prep time. Applications & Test Methods- Growth Promotion Testing
- Media Challenge Testing
- Suitability of Counting Methods
- Suitability of Sterility Tests
- Suitability of Tests for Specified Microorganisms
- Microbial Limits Testing
- Microbial Enumeration Testing
- Validation of Neutralisation Methods
- Methods requiring a low CFU concentration

